Summary
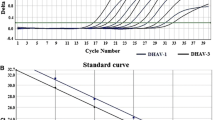
To examine the effect of Gankang Suppository on duck hepatitis B virus (DHBV), the serum biochemistry and hepatic histology in an animal model of DHBV infection, a model of DHBV infection was established by infecting 1-day-old Yingtaogu ducklings with DHBV-positive serum. The successful model was confirmed by PCR assay and 48 ducklings infected with DHBV were randomly divided into 3 groups: a Gankang Suppository treatment group, an acyclovir (ACV) group and a DHBV model group (control), with each group having 16 animals. All the animals were given the medicines for 4 weeks in a row. The serum of the animals was taken 14 and 28 days after the medication and 7 days after drug discontinuation. Real-time PCR was performed to detect the copy numbers of DHBV DNA in the serum. ALT and AST were dynamically monitored. The ducklings were sacrificed on the 7th day after the discontinuation of the treatment and livers were harvested and examined for inflammation and degeneration of liver cells by using HE staining. The results showed that on day 14, 28 after the treatment and day 7 after the withdrawal, the logarithmic values (log) of DHBV DNA copy numbers in ducklings of Gankang Suppository treatment group were significantly lower than that before the treatment (P=0.0092, P=0.0070, P=0.0080, respectively). Compared with DHBV model control group, the ALT level was significantly decreased (P=0.0020, P=0.0019, respectively) on day 28 after the treatment and on day 7 after the withdrawal. The AST level was also reduced on day 14 after the treatment (P=0.0298). Compared with the ACV control group, the level of ALT was lower on day 7 after the withdrawal (P=0.0016). Histologically, the hepatocyte swelling, vacuolous degeneration and acidophilic degeneration in Gankang Suppository treatment group were alleviated 7 days after the withdrawal as compared with model control group (P=0.0282, P=0.0084, P=0.0195, respectively). It is concluded that Gankang Suppository can effectively suppress DHBV replication, reduce the levels of serum ALT and AST and improve hepatic histology.
Similar content being viewed by others
References
Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepatitis, 2004,11(2):97–102
World Health Organization. Hepatitis B. World Health Organization Fact Sheet 204 (Revised October 2000). WHO Web site. 2000 http://who.int/inf-fs/en/fact204.html
Lok A S. Chronic hepatitis B. N Engl J Med, 2002,346(22):1682–1683
Yang D F, Tian D Y, Shen H X. The Effects and side effects of suppository Gankang on chronic HBV infection. Chin J Integr Tradit West Med Liver Dis (Chinese), 2002, 12(5):270–271
Bieche I, Olivi M, Champeme M H et al. Novel approach to quantitative polymerase chain reaction using real time detection: application to the detection of gene amplification in breast cancer. Int J Cancer, 1998,78(5):661–666
Lian Y X, Tan J, Deng Z D et al. Experimental study of shuangcao granule no. 1 on duck hepatitis B virus in ducklings. Chin J Integr Tradit West Med (Chinese), 2000, 20(7):530–532
Shead A, Vickery K, Pajkos A et al. Effects of phyllanthus plant extracts on duck hepatitis B virus in vitro and in vivo. Antiviral Res, 1992,18(2):127–138
Wen Y M. Study and application of therapeutic vaccine in Hepatitis B. Chin J Infect Dis (Chinese), 1996,149(3): 159–161
Cao H, Tao P. Anti-hepatitis B virus effects of lamivudine and other five drugs in vitro. Chin Med J (Chinese), 2001,81(16):1004–1007
Hantz O, Périgaud C, Borel C et al. The SATE pronucleotide approach applied to acyclovir: part II. Effects of bis (SATE) phosphotriester derivatives of acyclovir on duck hepatitis B virus replication in vitro and in vivo. Antiviral Res, 1999,40(3):179–187
Chen Y, Guo S, Qi Z et al. Experimental study on the effect of combination therapy with lamivudine and famciclovir against duck hepatitis B virus in vivo. Chin J Hepatol (Chinese), 2001,9(4):209–211
Zhang D F. Novel therapeutic strategies based on mechanism of clearance of HBV in body. Chin J Hepatol (Chinese), 2001,9(4):196–202
Zeng J, Tian D Y, Yang D F et al. Inhibitory effects in vitro of Gankang suppository on hepatitis B virus. Chin J Integr Tradit West Med Liver Dis (Chinese), 2004,14(1):39–41
Guidotti L G, Ando K, Hobbs M V et al. Cytotoxic T lymphocytes inhibits hepatitis B virus gene expression by a non-cytolytic mechanism in transgenic mice. Proc Natl Acad Sci USA, 1994,91:3764–3768
Webster G J, Reignat S, Maini M K et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology, 2000,32(5):1117–1124
Guidotti L G, Rocheford R, Chung L et al. Viral clearance without destruction of infected cells during acute HBV infection. Science, 1999,284(5415):825–829
Kroes B H, Beukelman C J, Vandenberg A J et al. Inhibition of human complement by β-glycyrrhetinic acid. Immunology, 1997,90(1):115–120
Kumada H. Long-term treatment of chronic hepatitis C with glycyrrhizin [Stronger Neo-Mimophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology, 2002,62(Suppl 1):94–100
Fujisawa Y, Sakamoto M, Matsushita M et al. Gly-cyrrhizin inhibits the lytic pathway of complement — possible mechanism of its anti-inflammatory effect on liver cells in viral hepatitis. Microbiol Immunol, 2000, 44(9):799–804
Author information
Authors and Affiliations
Corresponding author
Additional information
Hui LI, male, born in 1980, M.D., Ph.D.
This work was supported by a grant from the National Natural Science Foundation of China (No. 30471533).
Rights and permissions
About this article
Cite this article
Li, H., Tian, D., Wu, H. et al. The effect of Gankang Suppository on duck hepatitis B virus, serum biochemistry and liver histology in ducklings. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 28, 421–425 (2008). https://doi.org/10.1007/s11596-008-0410-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-008-0410-4