Summary
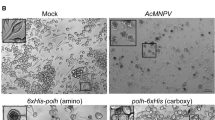
HBV polymerase has intrinsic RNA-dependent reverse transcriptase, DNA-dependent DNA polymerase as well as RNaseH activity. Analysis of HBV polymerase has been hampered for many years due to the inability to express functional enzyme in a recombinant system. To obtain active polymerase at a high level, we have taken advantage of baculovirus expression system. The gene of HBV polymerase was amplified by PCR and cloned into pFastBac Dual to construct the recombinant plasmid pFastbac Dual-pol. The recombinant donor plasmid, pFastbac Dual-pol, was constructed by inserting HBV polymerase gene into EcoRI and PstI sites controlled by polyhedrin promoter. The recombinant donor plasmid was transformed into DH10Bac competent cells for transposition. Recombinant bacmid was constructed by inserting of the mini-Tn7 element from the donor plasmid into the mini-attTn7 attachment site on the bacmid. The recombinant bacmid DNA was isolated and transfected into the Sf9 cells to produce the recombinant virus, and healthy insect Sf9 cells were infected with the recombinant virus containing HBV polymerse gene to express the target protein. HBV polymerse expressed in insect cells was analyzed by SDS-PAGE. PCR results showed recombinant donor plasmid, pFastbac Dual-pol, was constructed successfully. The recombinant hepatitis B virus polymerase was expressed in insect cells at high level. The recombinant hepatitis B virus polymerase should facilitate the analysis of HBV polymerase biological characteristics, allow the investigation for new anti-HBV drugs specifically blocking HBV polymerase.
Similar content being viewed by others
References
Ganem D. Hapadnaviridae and their replication. In Fields B N, Knipe D M, Howley P M. Fundamental Virology. 3rd ed. Philadelphia: Lippincott-Raven Publishers, 1996.1199–1233
Seeger C, Mason W S. Hepatitis B virus biology. Microbiol Mol Biol Rev, 2000, 64:51–68
Summers J, Mason W S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell, 1982, 29:403–415
Ganem D, Varmus H E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem, 1987,56:651–693
Bartenschlager R, Kuhn C, Schaller H. Expression of the P-protein of the human hepatitis B virus in a vaccine virus system and detection of the nucleocapsid-associated P-gene product by radiolabelling at newly introduced phosphorylation sites. Nucleic Acids Res, 1992,20:195–202
Radziwill G, Zentgraf H, Schaller H et al. The duck hepatitis B virus DNA polymerase is tightly associated with the viral core structure and unable to switch to an exogenous template. Virology, 1988,163:123–132
Lanford R E, Notvall L, Beames B. Nucleotide priming and reverse transcriptase activity of hepatitis B virus polymerase expressed in insect cells. J Virol, 1995,69:4431–4439
Seifer M, Standring D N. Recombinant human hepatitis B virus reverse transcriptase is active in the absence of the viral nucleocapsid or the replication origin, DR1. J Virol, 1993,67:4513–4520
Tavis J E, Ganem D. Expression of functional hepatitis B virus polymerase in yeast reveals it to be the sole viral protein required for correct initiation of reverse transcription. Proc Natl Acad Sci USA, 1993,90:4107–4111
Wang G H, Seeger C. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell, 1992,71:663–670
Lanford R E, Notvall L, Lee H et al. Transcomplementation of nucleotide priming and reverse transcription between independently expressed TP and RT domains of the hepatitis B virus reverse transcriptase. J Virol, 1997,71:2996–3004
Seifer M, Standring D N. Ribonucleoprotein complex formation by the human hepatitis B virus polymerase. Intervirology, 1995,38:295–303
Tavis J E, Ganem D. RNA sequences controlling the initiation and transfer of duck hepatitis B virus minus-strand DNA. J Virol, 1995,69:4283–4291
Galibert F, Mandart E, Fitoussi F et al. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature (London), 1979,281:646–650
Tani H, Limn C K, Yap C C et al. In vitro and in vivo gene delivery by recombinant baculoviruses. J Virol, 2003,77:9799–9808
Kost T A, Condreay J P, Jarvis D L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol, 2005,23:567–575
Huser A, Hafmann C. Baculovirus vectors: novel mammalian cell gene-delivery vehicles and their applications. Am J Pharmacogenomics, 2003,3:53–63
Bartenschlager R, Junker-Niepmann M, Schaller H. The P gene product of hepatitis B virus is required as a structural component for genomic RNA encapsidation. J Virol, 1990,64:5324–5332
Hirsch R C, Lavine J E, Chang L J et al. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as well as for reverse transcription. Nature (London), 1990,344:552–555
Bartenschlager R, Schaller H. The amino-terminal domain of the hepadnaviral P-gene encodes the terminal protein (genome-linked protein) believed to prime reverse transcription. EMBO J, 1988,7:4185–4192
Radziwill G, Tucker W, Schaller H. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J Virol, 1990,64:613–620
Wang G H, Seeger C. Novel mechanism for reverse transcription in hepatitis B viruses. J Virol, 1993,67:6507–6512
Tavis J E, Perri S, Ganem D E. Hepadnaviral reverse transcription initiates within a stem-loop of the RNA packaging signal and employs a novel strand transfer. J Virol, 1994,68:3536–3543
Author information
Authors and Affiliations
Corresponding author
Additional information
WANG Xiaoyan, female, born in 1980, M.D., Ph.D.
This project was supported by a grant from the National Natural Sciences Foundation of China (No. 30330680).
Rights and permissions
About this article
Cite this article
Wang, X., Gao, L., Deng, F. et al. High-level production of a functional recombinant hepatitis B virus polymerase in insect cells with a baculovirus expression system. J. Huazhong Univ. Sc. Technol. 27, 269–273 (2007). https://doi.org/10.1007/s11596-007-0313-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11596-007-0313-9