Abstract
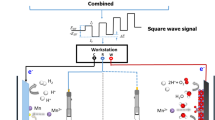
Influence mechanism of sulfide ions (S2-) during manganese electrodeposition from sulphate electrolyte was investigated. Under the experimental conditions, S2- ion concentration and electrolyte pH represented significant multiple effects on the cathode current efficiency. Scanning electron microscope (SEM) indicated that electrodeposition manganese layer (EML) became refinement as S2- ion concentration was increased. X-ray Diffraction (XRD) displayed that S2- ion had negligible influence on the preferred orientation of the crystalline layer of the EML. S2- ion had no influence on the chemical composition of EML. S2- ion could improve the corrosion resistance of EML. The interaction mechanism of S2- ion with manganese electrodeposition was systematically explored by analysing E-pH diagram, cyclic voltammetry curve, cathode polarization curve and electrochemical impedance method.
Similar content being viewed by others
References
Xing MW, Kong J, Dong J, et al. Thiophenic Sulfur Compounds Released During Coal Pyrolysis[J]. Environ. Eng. Sci., 2013, 30(6): 273–279
Ling YC, Liao JH. Matrix Effect on Supercritical Fluid Extraction of Organo Chlorine Pesticides from Sulfur-containing Soils[J]. J. Chromatogr. A, 1996, 754: 285–294
Taheri B, Abdollahy M, Tonkaboni SZS, et al. Dual Effects of Sodium Sulfide on the Flotation Behavior of Chalcopyrite: I. Effect of Pulp Potential[J]. Int. J. Min. Met. Mater., 2014, 21(5): 415–422
Salum MJG, Araujo AC de, Peres, AEC. The Role of Sodium Sulphide in Amine Flotation of Silicate Zinc Minerals[J]. Min. Eng., 1992, 5(3): 411–419
Poorkani M, Banisi S. Industrial Use of Nitrogen in Flotation of Molybdenite at the Sarcheshmeh Copper Complex[J]. Min. Eng., 2005, 18(7): 735–738
Hu YH, Sun W, Wang DZ. Electrochemistry of Flotation of Sulphide Minerals[M]. Beijing: Tsinghua University Press, 2009
Zhao Q, Liu WG, Wei DZ, et al. Effect of Copper Ions on the Flotation Separation of Chalcopyrite and Molybdenite Using Sodium Sulfide as a Depressant[J]. Min. Eng., 2018, 115: 44–52
Jandova J, Lisa K, Vu H, et al. Separation of Copper and Cobalt-nickel Sulfide Concentrates during Processing of Manganese Deep Ocean Nodules[J]. Hydrometallurgy, 2005, 77: 75–79
Yan S, Qiu YR. Preparation of Electronic Grade Manganese Sulfate from Leaching Solution of Ferromanganese Slag[J]. T. Nonferr. Metal. Soc., 2014, 24(11): 3 716–3 721
Ye MY, Yan PF, Ren J, et al. Removal of Metals from Lead-zinc Mine Tailings Using Bioleaching and Followed by Sulfde Precipitation[J]. Chemosphere, 2017, 185: 1 189–1 196
Lu JM, Dreisinger DB, Glückb T. Manganese Electrodeposition-A Literature Review[J]. Hydrometallurgy, 2014, 141: 105–116
Araujo JAM, Castro MMR, Lins VDFC. Reuse of Furnace Fines of Ferro Alloy in the Electrolytic Manganese Production[J]. Hydrometallurgy, 2006, 84: 204–210
Ding LF, Fan X, Du J, et al. Influence of Three N-based Auxiliary Additives during the Electrodeposition of Manganese[J]. Int. J. Min. Met. Mater., 2014, 130: 34–41
Padhy SK, Patnaik P, Tripathy BC, et al. Electrodeposition of Manganese Metal from Sulphate Solutions in the Presence of Sodium Octyl sulphate[J]. Hydrometallurgy, 2016, 165: 73–80
Lu JM, Dreisinger DB, Glückb T. Electrolytic Manganese Metal Production from Manganese Carbonate Precipitate[J]. Hydrometallurgy, 2016, 161: 45–53
Wei QF, Ren XL, Du J, et al. Study of the Electrodeposition Conditions of Metallic Manganese in an Electrolytic Membrane Reactor[J]. Min. Eng., 2010, 23: 578–586
Xu FY, Dan ZG, Zhao WN, et al. Electrochemical Analysis of Manganese Electrodeposition and Hydrogen Evolution from Pure Aqueous Sulfate Electrolytes with Addition of SeO2[J]. J. Electroanal. Chem., 2015, 741: 149–156
Xue JR, Wang S, Zhong H, et al. Influence of Sodium Silicate on Manganese Electrodeposition in Sulfate Solution[J]. T. Nonferr. Metal. Soc., 2016, 26: 1 126–1 137
Suh MS, Park CJ, Kwon HS. Effects of Plating Parameters on Alloy Composition and Microstructure of Sn-Bi Electrodeposits from Methane Sulphonate Bath[J]. Surf. Coat. Tech., 2006, 200: 3 527–3 532
Fan X, Xi SY, Sun DG, et al. Mn-Se Interactions at the Cathode Interface during the Electrolytic-manganese Process[J]. Hydrometallurgy, 2012, 127: 24–29
YB/T 051-2015. Electrolytic Manganese Metal-Black Metallurgical Industry Grade Standard in China[S], 2015
Song WM, Yang GR, Liao BB, et al. Effect of Cl− Concentration on the Corrosion Behavior of 16Mn Steel in Saturated H2S/CO2 Solution[J]. J.Wuhan Univ. Technol., 2018, 33(5): 1 205–1 215
Kan HM, Feng XJ, Wei XD, et al. Effects of Surfactants SDS and CTAB on Ni-SiC Deposition[J]. J.Wuhan Univ. Technol., 2018, 33(4): 836–842
Parada F, Jeffrey MI, Asselin E. Leaching Kinetics of Enargite in Alkaline Sodium Sulphide Solutions[J]. Hydrometallurgy, 2014, 146: 48–58
Li WF, Zhou YJ, Xue Y. Corrosion Behavior About Tubing Steel in Environment with High H2S and CO2 Content[J]. J.Wuhan Univ. Technol., 2013, 28(5): 1 038–1 043
Xue JR, Wang S, Zhong H, et al. Influence of Sodium Oleate on Manganese Electrodeposition in Sulfate Solution[J]. Hydrometallurgy, 2016, 160: 115–122
Feng QC, Wen SM, Zhao WJ, et al. Adsorption of Sulfide Ions on Cerussite Surfaces and Implications for Flotation[J]. App. Surf. Sci., 2016,: 365–372
Lai YQ, Liu FY, Li J, et al. Nucleation and Growth of Selenium Electrodeposition onto Tin Oxide Electrode[J]. J. Electroanal. Chem., 2010, 639: 187–192
De Oliveira EM, Carlos IA. Voltammetric and Morphological Characterization of Zinc Electrodeposition from Acid Electrolytes Containing Boric-polyalcohol Complexes[J]. J. Appl. Electrochem., 2008, 38: 1 203–1 210
Hosseini S, Kheawhom S, Soltani SM, et al. Electrochemical Reduction of Bicarbonate on Carbon Nanotube-supported Silver Oxide: An Electrochemical Impedance Spectroscopy Study[J]. J. Environ. Chem. Eng., 2018, 6: 1 033–1 043
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded partly by the National Natural Science Foundation of China (No. 21376273), the National Science and Technology Support Program of China(No. 2015BAB17B01), and the Open Research Fund of Hunan Provincial Science and the Technology Plan Project (No. MN2017K05), China
Rights and permissions
About this article
Cite this article
Xue, J., Zhong, H., Wang, S. et al. Influence Mechanism of Sulfide Ions during Manganese Electrodeposition. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 34, 1451–1459 (2019). https://doi.org/10.1007/s11595-019-2212-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-019-2212-x