Abstract
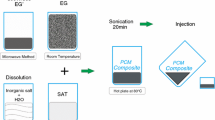
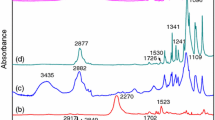
A 1-octadecanol (OD)/1,3:2,4-di-(3,4-dimethyl) benzylidene sorbitol (DMDBS)/expander graphite (EG) composite was prepared as a form-stable phase change material (PCM) by vacuum melting method. The results of field emission-scanning electron microscopy (FE-SEM) showed that 1-octadecanol was restricted in the three-dimensional network formed by DMDBS and the honeycomb network formed by EG. X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR) results showed that no chemical reaction occurred among the components of composite PCM in the preparation process. The gel-to-sol transition temperature of the composite PCMs containing DMDBS was much higher than the melting point of pure 1-octadecanol. The improvements in preventing leakage and thermal stability limits were mainly attributed to the synergistic effect of the three-dimensional network formed by DMDBS and the honeycomb network formed by EG. Differential scanning calorimeter (DSC) was used to determine the latent heat and phase change temperature of the composite PCMs. During melting and freezing process the latent heat values of the PCM with the composition of 91% OD/3% DMDBS/6% EG were 214.9 and 185.9 kJ·kg−1, respectively. Its degree of supercooling was only 0.1 °C. Thermal constant analyzer results showed that its thermal conductivity (κ) changed up to roughly 10 times over that of OD/DMDBS matrix.
Similar content being viewed by others
References
Kenisarin M M, Kenisarina K M. Form-stable Phase Change Materials for Thermal Energy Storage[J]. Renew. Sust. Energy Rev., 2012,16: 1 999∓2 040
Hasnain S M. Review on Sustainable Thermal Energy Storage Technologies, Part I: Heat Storage Materials and Techniques[J]. Energy Convers. Manage., 1998, 39: 1 127–1 138
Sharma A, Tyagi V V, Chen C R, et al. Review on Thermal Energy Storage with Phase Change Materials and Applications[J]. Renew. Sust. Energy Rev., 2009, 13: 318–345
Mettawee E, Assassa G. Experimental Study of a Compact PCM Solar Collector[J]. Energy, 2006, 31: 2 958–2 968
Francis A, Neil H, Philip E, et al. A Review of Materials, Heat Transfer and Phase Change Problem Formulation for Latent Heat Thermal Energy Storage Systems (LHTESS)[J]. Renew. Sust. Energy Rev., 2010, 14: 615–628
Cabeza L F, Castell A, Barreneche C, et al. A. I. Fernandez, Materials Used as PCM in Thermal Energy Storage in Buildings: a Review[J]. Renew. Sust. Energy Rev., 2011, 15: 1 675–1 695
Mohammed M F, Amar M K, Siddique A K R, et al. A Review on Phase Change Energy Storage: Materials and Applications[J]. Energy Convers. Manage, 2004, 45: 1 597–1 615
Tunçbilek K, Sari A, Tarhan S, et al. Lauric and Palmitic Acids Eutectic Mixture as Latent Heat Storage Material for Low Temperature Heating Applications[J]. Energy, 2005, 30: 677–692
Zalba B, Marin J M, Cabeza L F, et al. Review on Thermal Energy Storage with Phase Change: Materials, Heat Transfer Analysis and Applications[J]. Appl. Therm. Eng., 2003, 23: 251–283
Ahmet S, Cemil A, Alper B. Development, Characterization, and Latent Heat Thermal Energy Storage Properties of Neopentyl Glycol-Fatty Acid Esters as New Solid-Liquid PCMs[J]. Ind. Eng. Chem. Res., 2013, 52: 18 269–18 275
Regin A F, Solanki A C, Saini J S. Heat Transfer Characteristics of Thermal Energy Storage System Using PCM Capsules: A Review[J]. Renew. Sust. Energy Rev., 2008, 12: 2 438–2 458
Sarier N, Onder E. Organic Phase Change Materials and Their Textile Applications: An Overview[J]. Thermochim. Acta, 2012, 540: 7–60
Hasan A, Sayigh A A. Some Fatty Acids as Phase Change Thermal Energy Storage Materials[J]. Renew. Energy, 1994, 4: 69–76
Sharma A, Tyagi V V, Chen C R, et al. Review on Thermal Energy Storage with Phase Change Materials and Applications[J]. Renew. Sust. Energy Rev., 2009, 13: 318–345
Zhang L, Zhu J Q, Zhou W B, et al. Characterization of Polymethyl Methacrylate/Polyethylene Glycol/Aluminum Nitride Composite as Form-stable Phase Change Material Prepared by In Situ Polymerization Method[J]. Thermochim. Acta, 2011, 524: 128–134
Yavari F, Fard H R, Pashayi K, et al. Enhanced Thermal Conductivity in a Nanostructured Phase Change Composite due to Low Concentration Graphene Additives[J]. Phys. Chem., 2011, 115: 8 753–8 758
Sari A, Karaipekli A. Thermal Conductivity and Latent Heat Thermal Energy Storage Characteristics of Paraffin/Expanded Graphite Composite as Phase Change Material[J]. Appl. Thermal Eng., 2007, 27: 1 271–1 277
Molefia J A, Luyt A S, Krupa I. Comparison of LDPE, LLDPE and HDPE as Matrices for Phase Change Materials Based on a Soft Fischer-Tropsch Paraffin Wax[J]. Thermochim. Acta, 2010, 500: 88–92
Alkan C, Sari A. Poly(ethylene glycol)/Acrylic Polymer Blends for Latent Heat Thermal Energy Storage[J]. AIChE J., 2006, 52: 3 310–3 314
Niu L B, Bai G Y, Song J. 1,3:2,4-di-(3,4-dimethyl) Benzylidene Sorbitol Organogels Used as Phase Change Materials: Solvent Effects on Structure, Leakage and Thermal Performance[J]. RSC Advances, 2015, 5: 21 733–21 739
Zhang L, Zhu J Q, Zhou W B, et al. Thermal and Electrical Conductivity Enhancement of Graphite Nanoplatelets on Form-stable Polyethylene Glycol/Polymethyl Methacrylate Composite Phase Change Materials[J]. Energy, 2012, 39: 294–302
Tian T, Song J, Niu L B, et al. Preparation and Properties of 1-tetradecanol/1,3:2,4-di-(3,4-dimethyl) Benzylidene Sorbitol Gelatinous Form-stable Phase Change Materials[J]. Thermochim. Acta, 2013, 554: 54–58
Takahashi A, Sakai M, Kato T. Melting Temperature of Thermally Reversible Gel. VI. Effect of Branching on the Sol-gel Transition of Polyethylene Gels[J]. Polym. J., 1980, 12: 335–341
Beginn U. Applicability of Frozen Gels from Ultra High Molecular Weight Polyethylene and Paraffin Waxes as Shape Persistent Solid/Liquid Phase Change Materials[J]. Macromol. Mater. Eng., 2003, 288: 245–251
Edwards W, Lagadec C A, Smith D K. Solvent-Gelator Interactionsusing Empirical Solvent Parameters to Better Understand the Self-assembly of Gel-phase Materials[J]. Soft Mater., 2011, 7(1): 110–117
Ventolà L, Ramirez M, Calvet T, et al. Polymorphism of N-Alkanols: 1-Heptadecanol, 1-Octadecanol, 1-Nonadecanol, and 1-Eicosanol[J]. Chem. Mater., 2002, 14: 508–517
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by Science and Technology Support Program of Hubei Province of China (No. 2015BAA111)
Rights and permissions
About this article
Cite this article
Xu, J., Cheng, X., Li, Y. et al. Preparation and Properties of l-octadecanol/1,3:2,4-di-(3,4-dimethyl) Benzylidene Sorbitol/Expanded Graphite Form-stable Composite Phase Change Material. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 34, 728–735 (2019). https://doi.org/10.1007/s11595-019-2110-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-019-2110-2