Abstract
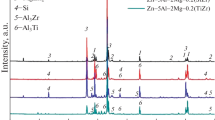
The effects of Mg on the microstructure and growth kinetics of the hot-dip Zn-22.3Al-1.1Si-x Mg (x = 0, 0.2, 0.4, and 0.6) coatings were investigated in detail. Scanning electron microscopy with energy dispersive spectroscopy and X-ray diffraction studies revealed the presence of η-Zn, α-Al, Zn/Al eutectoid, (Si), Al/Si eutectic, and Zn/Al/Mg2Zn11 ternary eutectic on the top surface of these Mg-containing coatings. Especially, a small amount of MgZn2 phase appears in top surface of Zn-22.3Al-1.1Si-0.6Mg coating. Five phases are found in the alloy layers, i e, Fe2Al5, FeAl3, τ5C, τ5H, and τ1. The addition of 0.2% Mg can delay the emergence of FeAl3 phase. When the Mg content is more than 0.2%, the outer layers of coating change from τ5C to τ5H phase. The growth of the inhibition layer is diffusion controlled for various Mg content baths. Mg improves the corrosion resistance of the Mg-containing coatings, and the Zn-22.3Al-1.1Si-0.6Mg coating possesses the highest protective properties.
Similar content being viewed by others
References
Li SW, Gao B, Yin SH, et al. The Effects of RE and Si on the Microstructure and Corrosion Resistance of Zn–6Al–3Mg Hot Dip Coating[ J]. Applied Surface Science, 2015, 357: 2 004–2 012
Padilla V, Ghods P, Alfantazi A. Effect of De–icing Salts on the Corrosion Performance of Galvanized Steel in Sulphate Contaminated Soil[J]. Construction & Building Materials, 2013, 40: 908–918
Vagge ST, Raja VS, Narayanan RG. Effect of Deformation on the Electrochemical Behavior of Hot–dip Galvanized Steel Sheets[J]. Applied Surface Science, 2007, 253(20): 8 415–8 421
Mandal GK, Das SK, Balasubramaniam R, et al. Evolution of Microstructures of Galvanised and Galvannealed Coatings Formed in 0.2 wt% Aluminium–Zinc Bath[J]. Materials Science and Technology, 2011, 27(8): 1 265–1 270
Dutta M, Halder AK, Singh SB. Morphology and Properties of Hot Dip Zn–Mg and Zn–Mg–Al Alloy Coatings on Steel Sheet[J]. Surface and Coatings Technology, 2010, 205(7): 2 578–2 584
Li SW, Gao B, Tu GF, et al. Effects of Magnesium on the Microstructure and Corrosion Resistance of Zn–55Al–1.6Si Coating[J]. Construction & Building Materials, 2014, 71: 124–131
Liu W, Li MC, Luo Q, et al. Influence of Alloyed Magnesium on the Microstructure and Long–term Corrosion Behavior of Hot–dip Al–Zn–Si Coating in NaCl Solution[J]. Corrosion Science, 2016, 104: 217–226
Prosek T, Nazarov A, Goodwin F, et al. Improving Corrosion Stability of Zn–Al–Mg by Alloying for Protection of Car Bodies[J]. Surface and Coatings Technology, 2016, 36: 439–447
Oh MS, Kim SH, Kim JS, et al. Surface and Cut–edge Corrosion Behavior of Zn–Mg–Al Alloy–coated Steel Sheets as a Function of the Alloy Coating Microstructure[J]. Metals and Materials International, 2016, 22(1): 26–33
Jiang L, Volovitch P, Wolpers M, et al. Activation and Inhibition of Zn–Al and Zn–Al–Mg Coatings on Steel by Nitrate in Phosphoric Acid Solution[J]. Corrosion Science, 2012, 60: 256–264
Commenda C, Pühringer J. Microstructural Characterization and Quantification of Zn–Al–Mg Surface Coatings[J]. Materials Characterization, 2010, 61(10): 943–951
Yu KC, Li J, Liu X, et al. Microstructure of Hot–dip Galvanized Zn–Al–Mg Alloy Coating[J]. Journal of Shanghai Jiaotong University (Sci.), 2012, 17: 663–667
Volovitch P, Allely C, Ogle K. Understanding Corrosion via Corrosion Product Characterization: I. Case Study of the Role of Mg Alloying in Zn–Mg Coating on Steel[J]. Corrosion Science, 2009, 51(6): 1 251–1 262
Lee JY, Yun YS, Kim WT, et al. Twinning and Texture Evolution in Binary Mg–Ca and Mg–Zn Alloys[J]. Metals and Materials International, 2014, 20(5): 885–891
Cho JH, Kim HW, Lim CY, et al. Microstructure and Mechanical Properties of Al–Si–Mg Alloys Fabricated by Twin Roll Casting and Subsequent Symmetric and Asymmetric Rolling[J]. Metals and Materials International, 2014, 20(4): 647–652
Mondolfo LF. Aluminum Alloys: Structure and Properties[M]. Elsevier, 2013
Yeh MS, Chang JC, Chuang TH. Stress Corrosion Cracking of a Superplastic and Nonsuperplastic Zn–22.3Al Alloy in 3% NaCl Solution[J]. Journal of Materials Engineering and Performance, 1999, 8(2): 219–224
Ranjan M, Tewari R, Van Ooij WJ, et al. Effect of Ternary Additions on the Structure and Properties of Coatings Produced by a High Aluminum Galvanizing Bath[J]. Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science, 2004, 35(12): 3 707–3 720
Wei DS, Tu H, Zhou SJ, et al. Effect of Silicon on the Reaction between Solid Iron and Liquid Zn–22.3wt% Al Bath[J]. Surface and Coatings Technology, 2016, 305: 29–35
Li Q, Zhao YZ, Luo Q, et al. Experimental Study and Phase Diagram Calculation in Al–Zn–Mg–Si Quaternary System[J]. Journal of Alloys and Compounds, 2010, 501(2): 282–290
Honda K, Yamada W, Ushioda K. Solidification Structure of the Coating Layer on Hot–dip Zn–11%Al–3%Mg–0.2%Si–Coated Steel Sheet[J]. Materials Transactions, 2008, 49(6): 1 395–1 400
De Bruycker E, Zermout Z, De Cooman BC. Zn–Al–Mg Coatings: Thermodynamic Analysis and Microstructure Related Properties[J]. Materials Science Forum, 2007, 539: 1 276–1 281
Shahverdi HR, Ghomashchi MR, Shabestari S, et al. Microstructural Analysis of Interfacial Reaction Between Molten Aluminium and Solid Iron[J]. Journal of Materials Processing Technology, 2002, 124(3): 345–352
Corby RN, Black PJ. The Structure of α–(AlFeSi) by Anomalous–dispersion Methods[J]. Acta Crystallographica, Section B: Structural Crystallography and Crystal Chemistry, 1977, 33(11): 3 468–3 475
Pontevichi S, Bosselet F, Barbeau F, et al. Solid–liquid Phase Equilibria in the Al–Fe–Si System at 727 °C[J]. Journal of Phase Equilibria and Diffusion, 2004, 25(6): 528–537
Liu ZK, Chang YA. Thermodynamic Assessment of the Al–Fe–Si System[ J]. Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science, 1999, 30(4): 1 081–1 095
Raynor GV, Rivlin VG. Phase Equilibria in Iron Ternary Alloys–A Critical Assessment of the Experimental Literature[J]. The Institute of Metals, 1 Carlton House Terrace, London SW 1 Y 5 DB, UK, 1988
Phelan D, Xu BJ, Dippenaar R. Formation of Intermetallic Phases on 55 wt.% Al–Zn–Si Hot Dip Strip[J]. Materials Science & Engineering, A: Structural Materials: Properties, Microstructure and Processing, 2006, 420(1): 144–149
Du Y, Schuster JC, Liu ZK, et al. A Thermodynamic Description of the Al–Fe–Si System over the Whole Composition and Temperature Ranges via a Hybrid Approach of CALPHAD and Key Experiments[J]. Intermetallics, 2008, 16(4): 554–570
Eleno L, Vezely J, Sundman B, et al. Assessment of the Al Corner of the Ternary Al–Fe–Si System[J]. Materials Science Forum, 2010, 649: 523–528
Tu H, Song YY, Liu Y, et al. Effect of Silicon on Microstructure and Growth Kinetics of Hot–Dip Galvanized ZnAl4 Coatings[J]. Materials Science and Engineering of Powder Metallurgy, 2015, 20(6): 815–821
Tang NY, Liu YH. Discussion of “Interfacial Layer in Coatings Produced in Molten Zn–Al Eutectoid Alloy Containing Si”[J]. Metallurgical and Materials Transactions A: Physical Metallurgy and Materials Science, 2005, 36(9): 2 541–2 544
Rivlin VG. and Raynor GV. 4: Critical Evaluation of Constitution of Aluminium–Iron–Silicon System[J]. International Metals Reviews, 1981, 26(1): 133–152
Haitani T, Tamura Y, Motegi T, et al. Solubility of Iron in Pure Magnesium and Cast Structure of Mg–Fe Alloy[J]. Materials Science Forum, 2003, 419: 697–702
Prosek T, Nazarov A, Bexell U, et al. Corrosion Mechanism of Model Zinc–Magnesium Alloys in Atmospheric Conditions[J]. Corrosion Science, 2008, 50(8): 2 216–2 231
Schürz S, Luckeneder G, Fleischanderl M, et al. Chemistry of Corrosion Products on Zn–Al–Mg Alloy Coated Steel[J]. Corrosion Science, 2010, 52(10): 3 271–3 279
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the National Natural Science Foundation of China (Nos. 51271040 and 51271041) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD)
Rights and permissions
About this article
Cite this article
Tu, H., Wei, D., Zhou, S. et al. Effect of Mg on Microstructure and Growth Kinetics of Zn-22.3Al-1.1Si Coating. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 34, 373–382 (2019). https://doi.org/10.1007/s11595-019-2062-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-019-2062-6