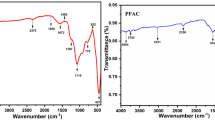
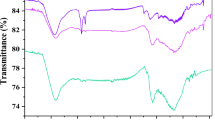
Abstract
The modification of activated carbon with persimmon tannin and its application for the removal of Pb(II) ions were carried out by batch method. The effects of solution pH, contact time, temperature and initial concentration on the immobilization of persimmon tannin were studied. The experimental results showed that the experimental data of persimmon tannin and Pb(II) fitted better by Langmuir adsorption isotherm model and pseudo-second order model. The adsorption capacities of adsorbents for persimmon tannin and Pb(II) were calculated from the Langmuir isotherm model, and found to be 42.97 and 12.40 mg/g at optimum pH, respectively. It was noted that the adsorbent exhibited the best adsorption property for Pb(II) when 1.0 g activated carbon was modified by 17.32 mg persimmon tannin. The modified activated carbon is more effective than the plain activated carbon, and it is expected to be an economic and effective adsorbent for the disposal of wastewater containing Pb(II) ions.
Similar content being viewed by others
References
Shao WJ, Chen LH, Lü LL, et al. Removal of Lead (II) from Aqueous Solution by a New Biosorption Material by Immobilizing Cyanex272 in Cornstalks[J]. Desalination, 2011, 265: 177–183
Yurtsever M, Sengil IA. Biosorption of Pb(II) Ions by Modified Quebracho Tannin Resin[J]. J. Hazard. Mater., 2009, 163: 58–64
Liu C, Bai R, Ly QS. Selective Removal of Copper and Lead Ions by Diethylenetriamine-functionalized Adsorbent: Behaviors and Mechanisms[J]. Water Res., 2008, 42: 1 511–1 522
Singh CK, Sahu JN, Mahalik KK, et al. Studies on the Removal of Pb(II) from Wastewater by Activated Carbon Developed from Tamarind Wood Activated with Sulphuric Acid[J]. J. Hazard. Mater., 2008, 153: 221–228
Singh V, Tiwari S, Kumar Sharma A, et al. Removal of Lead from Aqueous Solutions using Cassia Grandis Seed Gum-graft-poly (methylmethacrylate)[J]. J.Colloid Interface Sci., 2007, 316: 224–232
Yin CY, Aroua MK, Ashri WM, et al. Review of Modifications of Activated Carbon for Enhancing Contaminant Uptakes from Aqueous Solutions[J]. Sep.Purif.Technol., 2007, 52: 403–415
Mohan D, Chander S. Single Component and Multi-component Adsorption of Metal Ions by Activated Carbons[J]. Colloids Surf., 2001, 177A: 183–196
Ozer A, Tanyildizi MS, Tumen F. Study of Cadmium Adsorption from Aqueous Solution on Activated Carbon from Sugar Beet Pulp[J]. Environ. Technol., 1998, 19: 1 119–1 125
Yin C, Aroua M, Daud W. Modification of Granular Activated Carbon using Low Molecular Weight Polymer for Enhanced Removal of Cu2+ from Aqueous Solution[J]. Water Sci. Technol., 2007, 56: 95–101
Ücer A, Uyanik A, Aygün SF. Adsorption of Cu(II), Cd(II), Zn(II), Mn(II) and Fe(III) Ions by Tannic Acid Immobilized Activated Carbon[J]. Sep.Purif.Technol., 2006, 47: 113–118
Alves J, Freire C, Castro B, et al. Anchoring of Organic Molecules onto Activated Carbon[J]. Colloids Surf., 2001, 189A: 75–84
Nakajima A, Baba Y. Mechanism of Hexavalent Chromium Adsorption by Persimmon Tannin Gel[J]. Water Res., 2004, 38: 2 859–2 864
Bailey SE, Olin TJ, Bricka RM, et al. A review of Potentially Low-cost Sorbents for Heavy Metals[J]. Water Res., 1999, 33(11): 2 469–2 479
Gaballah I, Kilbertus G. Recovery of Heavy Metal Ions Through Decontamination of Synthetic Solutions and Industrial Effluents using Modified barks[J]. J. Geochem. Exp., 1998, 62: 241–286
Yu B, Zhang Y, Shukla A, et al. The Removal of Heavy Metal from Aqueous Solutions by Sawdust Adsorption-removal of Copper[J]. J. Hazardous. Mater., 2000, 80: 33–42
Rahim AA, Rocca E, Steinmetz J, et al. Mangrove Tannins and Their Flavanoid Monomers as Alternative Steel Corrosion Inhibitors in Acidic Medium[J]. Corros. Sci., 2007, 49: 402–417
Lima L, Olivares S, Martinez F, et al. Use of Immobilized Tannin Adsorbent for Removal of Cr(VI) from Water[J]. J. Radioanal.Nucl. Chem., 1998, 231: 35–40
Hemingway R, Laks P. Plant Polyphenols[M]. New York: Plenum Press, 1992: 421–450
Zhan XM, Zhao X. Mechanism of Lead Adsorption from Aqueous Solutions using an Adsorbent Synthesized from Natural Condensed Tannin[J]. Water Res., 2003, 37: 3 905–3 912
Nakajima A, Sakaguchi T. Uptake and Recovery of Gold by Immobilized Persimmon Tannin[J]. J. Chem. Tech. Biotechnol., 1993, 57:321–326
Freundlich HMF. Over the Adsorption in Solution[J]. J. Phys. Chem., 1906, 57: 385–470
Langmuir, I. The Adsorption of Gases on Plane Surface of Glass, Mica and Platinum[J]. J. Am. Chem. Soc., 1918, 40: 1 361–1 403
Lagergren S. About the Theory of the So-called Adsorption of Soluble Substances[J]. Vetensk.akad. Handl., 1898, 24: 1–39
Ho Y, Chian C, Hsu Y. Sorption Kinetics for Dye Removal from Aqueous Solution using Activated Clay[J]. J. Sep. Sci. Technol., 2001, 36: 2 473–2 48
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the National Military-funded Projects of China (No. 9140A12011108QT6912)
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, F., Wan, T. et al. Enhanced adsorption of Pb(II) ions from aqueous solution by persimmon tannin-activated carbon composites. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 28, 650–657 (2013). https://doi.org/10.1007/s11595-013-0746-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-013-0746-x