Abstract
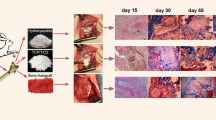
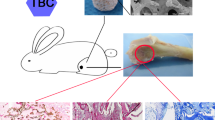
HA/TCP and HA rods (ϕ5 mm×10 mm) were made for implantation in New Zealand white rabbit with different condition. Sixty three rabbit were divided into three groups: group 1 (n=18), group 2 (n=27) and group 3 (n=18). In group 1, 10 mm radius was defected, and one HA/TCP rod was implanted in the muscle a distant away from the bone defect area. In group 2, also, 10 mm radius was defected, one HA rod was implanted in the muscle a distant away from the bone defect area. In group 3, two HA/TCP rods were implanted in the dorsal muscle of the rabbit with bone intact. Histological observation showed that in group 1, some new bone was found only two months after implantation (n=2), and obvious immature woven bone could be observed in these bioceramics from the 3rd month on. However, in group 3, bone began to be found 6 months after implantation (n=2). In group 2, we could not find any bone tissue up to 9 month’s observation. These results suggest that, first, the bone defect model could significantly accelerate bone formation at non-osseous sites in rabbits; second,. HA/TCP bioceramics were confirmed with osteoinductive property while HA bioceramics without osteoinductive property nearly. Thus, bone defect might be a good animal model for further researches for osteoinductive bioceramics.
Similar content being viewed by others
References
Yamasaki H. Heterotopic Bone Formation around Porous Hydroxyapatite Ceramics in the Subcutis of Dogs[J]. J. Oral. Biol. (Japan), 1990, 32: 190–192
Zhang XD, Zou P, Wu C, et al. A Study of Porous Block HA Ceramics and its Osteogeneses[J]. In: Bioceramics and the Human Body. ED by Ravaglioli and Krajewski.Elsevier Applied Science, 1991, 408–415
Ripamonti U. The Morphogenesis of Bone in Replicas of Porous Hydroxyapatite Obtained from Conversion of Calcium Carbonate Exoskeletons of Coral[J]. J. Bone Joint Surg., 1991, 73: 692–703
Ripamonti U. Osteoinduction in Porous Hydroxyapatite Implanted in Heterotopic Sites of Different Animal Models[J]. Biomaterials, 1996; 17: 31–35
XD Zhang, HP Yuan, K de Groot. Calcium Phosphate Biomaterials with Intrinsic Osteoinductivity[C]. The 6 th World Biomater Congress, 2000
HP Yuan, ZJ Yang, P Zou, et al. An Investigation of the Osteoinduction of Synthetic Porous Phase-Pure Hydroxyapatite Ceramic[J]. Biomed. Eng. Appl. Basis Comm., 1997, 9: 268–273
HP Yuan, ZJ Yang, YB Li, et al. Osteoinduction by Calcium Phosphate Biomaterials[J]. J. Mater. Sci: Mater. Med., 1998, 9: 723–726
Kurashina K, Kurita H, Wu Q, et al. Ectopic Osteogenesis with Biphasic Ceramics of Hydroxyapatite and Tricalcium Phosphate in Rabbits[J]. Biomaterials, 2002, 23: 407–412
HP Yuan, M Van De Doel, SH Li, et al. A Comparison of the Osteoinductive Potential of Two Calcium Phosphate Ceramics Implanted Intramuscularly in Goats[J]. J. Mater. Sci: Mater. Med., 2002, 13: 1 271–1 275
R Dimitriou, E Tsiridis, PV Giannoudis. Current Concepts of Molecular Aspects of Bone Healing[J]. Injury, 2005, 36: 1 392–1 404
GS Themistocleous, SE Kontou, P Lembessis, et al. Skeletal Growth Factor Involvement in the Regulation of Fracture Healing Process[J]. In vivo, 2003, 17: 489–503
Cho TJ, Gerstenfeld LC, Einhorn TA. Differential Temporal Expression of Members of the Transforming Growth Factor Beta Superfamily during Murine Fracture Healing[J]. J. Bone Miner. Res., 2002, 17: 513–520
AH Reddi. Bone Morphogenetic Proteins: From Basic Science to Clinical Applications[J]. J. Bone Joint Surg.[Am], 2001, 83: S1–S6
T Sakou. Bone Morphogenetic Proteins: From Basic Studi — esto Clinical Approaches[J]. Bone, 1998, 22(6): 591–603
H Cheng, W Jiang, FM Philips, et al. Osteogenic Activity of the Fourteen Types of Human Bone Morphogenetic Proteins (BMPs)[J]. J. Bone Joint Surg.[Am], 2003, 85-A: 1 544–1 552
MP Bostrom. Expression of Bone Morphogenetic Proteins in Fracture Healing[J]. Clin. Orthop., 1998, 355S: 116–123
Mundy GR. Regulation of Bone Formation by Bone Morphogenetic Proteins and other Growth Factors[J]. Clin. Orthop. Relat. Res., 1996, 324: 24–28
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the National Basic Research Program of China (973 Program) (No. 2005CB623900-G)
Rights and permissions
About this article
Cite this article
Cheng, L., Ye, F., Lu, X. et al. Tissue response of an osteoinductive bioceramic in bone defect rabbit model. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 25, 28–31 (2010). https://doi.org/10.1007/s11595-010-1028-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-010-1028-5