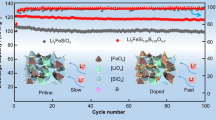
Abstract
Reasonable design of cathode-electrolyte interface (CEI) with high electrochemical stability and high ionic conductivity is crucial for improving the electrochemical performance of cathode materials. Herein, lithium difluoro(oxalate)borate (LiDFOB) is used as an efficient electrolyte additive to form a robust, dense, and conductive CEI on LiFePO4 cathode, which significantly enhances the rate capability and cycling stability. Specifically, the introduction of LiDFOB promotes the formation of a fluoride (F)- and boron (B)-rich CEI, effectively enhancing ion transport kinetics. Simultaneously, the generated CEI exhibits excellent stability, efficiently suppressing the continuous decomposition of the electrolyte and structural damage of LiFePO4 cathode over prolonged cycling. Consequently, the LiFePO4 cathode with LiDFOB additive exhibits a highly reversible capacity of 133.4 mAh g−1 at 0.5 C and an excellent capacity retention of 94.4% over 400 cycles, which is superior to LiFePO4 without additive (only 66.1% capacity retention). This work confirms the promising potential of LiDFOB as a multifunctional electrolyte additive and provides new insights for constructing high-performance CEI.





Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
References
Ni S, Liu J, Chao D et al (2019) Vanadate-based materials for Li-ion batteries: the search for anodes for practical applications. Adv Energy Mater 9(14):1803324
Choi JW, Aurbach D (2016) Promise and reality of post-lithium-ion batteries with high energy densities. Nat Rev Mater 1(4):16013
Goodenough JB, Park K-S (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135(4):1167–1176
Shu H, Wang X, Wu Q et al (2013) Improved electrochemical performance of LiFePO4/C cathode via Ni and Mn co-doping for lithium-ion batteries. J Power Sources 237:149–155
Shu H, Chen M, Fu Y et al (2014) Improvement of electrochemical performance for spherical LiFePO4 via hybrid coated with electron conductive carbon and fast Li ion conductive La0.56Li0.33TiO3. J Power Sources 252:73–78
Shu H, Wang X, Wen W et al (2013) Effective enhancement of electrochemical properties for LiFePO4/C cathode materials by Na and Ti co-doping. Electrochim Acta 89:479–487
Larcher D, Tarascon JM (2015) Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 7(1):19–29
Liu Q, Li Z-F, Liu Y et al (2015) Graphene-modified nanostructured vanadium pentoxide hybrids with extraordinary electrochemical performance for Li-ion batteries. Nat Commun 6(1):6127
Du M, Guo J-Z, Zheng S-H et al (2023) Direct reuse of LiFePO4 cathode materials from spent lithium-ion batteries: extracting Li from brine. Chin Chem Lett 34(6):107706
Dou W, Wan G, Liu T et al (2023) Conductive composite binder for recyclable LiFePO4 cathode. Chin Chem Lett. https://doi.org/10.1016/j.cclet.2023.109389
Wang L, Qiu J, Wang X et al (2022) Insights for understanding multiscale degradation of LiFePO4 cathodes. eScience 2(2):125–137
Park S, Jeong SY, Lee TK et al (2021) Replacing conventional battery electrolyte additives with dioxolone derivatives for high-energy-density lithium-ion batteries. Nat Commun 12(1):838
Wang XT, Gu ZY, Ang EH et al (2022) Prospects for managing end-of-life lithium-ion batteries: present and future. Interdiscip Mater 1(3):417–433
Zhang Z, Zhao D, Xu Y et al (2022) A review on electrode materials of fast-charging lithium-ion batteries. Chem Rec 22(10):e202200127
Yoo DJ, Liu Q, Cohen O et al (2023) Rational design of fluorinated electrolytes for low temperature lithium-ion batteries. Adv Energy Mater 13(20):2204182
Qian Y, Hu S, Zou X et al (2019) How electrolyte additives work in Li-ion batteries. Energy Storage Mater 20:208–215
Haregewoin AM, Wotango AS, Hwang B-J (2016) Electrolyte additives for lithium ion battery electrodes: progress and perspectives. Energy Environ Sci 9(6):1955–1988
Sun J, Zhang S, Zhang Q et al (2022) Unshackling the reversible capacity of SiOx/graphite-based full cells via selective LiF-induced lithiation. Sci China Mater 65(9):2335–2342
Beltrop K, Klein S, Nölle R et al (2018) Triphenylphosphine oxide as highly effective electrolyte additive for graphite/NMC811 lithium ion cells. Chem Mater 30(8):2726–2741
Zhao Q, Guo C, Lu Y et al (2016) Rechargeable lithium batteries with electrodes of small organic carbonyl salts and advanced electrolytes. Ind Eng Chem Res 55(20):5795–5804
Younesi R, Veith GM, Johansson P et al (2015) Lithium salts for advanced lithium batteries: Li–metal, Li–O2, and Li–S. Energy Environ Sci 8(7):1905–1922
Dong Q, Guo F, Cheng Z et al (2019) Insights into the dual role of lithium difluoro(oxalato)borate additive in improving the electrochemical performance of NMC811||graphite cells. ACS Appl Energy Mater 3(1):695–704
Lindgren F, Xu C, Niedzicki L et al (2016) SEI formation and interfacial stability of a Si electrode in a LiTDI-salt based electrolyte with FEC and VC additives for Li-ion batteries. ACS Appl Mater Interf 8(24):15758–15766
Zhou K, Yang H, Zhou J et al (2023) Abnormal electrode behavior of graphite anode operating with the electrolyte containing vinylene carbonate and ethylene sulfite additives. Electrochim Acta 462:142768
Rezqita A, Sauer M, Foelske A et al (2017) The effect of electrolyte additives on electrochemical performance of silicon/mesoporous carbon (Si/MC) for anode materials for lithium-ion batteries. Electrochim Acta 247:600–609
Xu M, Zhou L, Hao L et al (2011) Investigation and application of lithium difluoro(oxalate)borate (LiDFOB) as additive to improve the thermal stability of electrolyte for lithium-ion batteries. J Power Sources 196(16):6794–6801
Applestone D, Manthiram A (2012) Symmetric cell evaluation of the effects of electrolyte additives on Cu2Sb–Al2O3–C nanocomposite anodes. J Power Sources 217:1–5
Lin L, Yang K, Tan R et al (2017) Effect of sulfur-containing additives on the formation of a solid-electrolyte interphase evaluated by in situ AFM and ex situ characterizations. J Mater Chem A 5(36):19364–19370
Wang P-L, Sun X-Z, An Y-B et al (2022) Additives to propylene carbonate-based electrolytes for lithium-ion capacitors. Rare Met 41(4):1304–1313
He R, Peng F, Dunn WE (2017) Chemical and electrochemical stability enhancement of lithium bis(oxalato)borate (LiBOB)-modified solid polymer electrolyte membrane in lithium ion half-cells. Electrochim Acta 246:123–134
Bian X, Ge S, Pang Q et al (2018) A novel lithium difluoro(oxalate) borate and lithium hexafluoride phosphate dual-salt electrolyte for Li-excess layered cathode material. J Alloys Compd 736:136–142
Zhang J, Zhang H, Deng L et al (2023) An additive-enabled ether-based electrolyte to realize stable cycling of high-voltage anode-free lithium metal batteries. Energy Storage Mater 54:450–460
Allen JL, Han S-D, Boyle PD (2011) Crystal structure and physical properties of lithium difluoro(oxalato)borate (LiDFOB or LiBF2Ox). J Power Sources 196(22):9737–9742
Kim J-K, Jeong SM (2020) Physico-electrochemical properties of carbon coated LiFePO4 nanoparticles prepared by different preparation method. Appl Surf Sci 505:144630
Lai A, Chu Y, Jiang J et al (2022) Self-restriction to form in-situ N, P co-doped carbon-coated LiFePO4 nanocomposites for high-performance lithium ion batteries. Electrochim Acta 414:140161
Wang Y, Wang X, Jiang A et al (2019) A versatile nitrogen-doped carbon coating strategy to improve the electrochemical performance of LiFePO4 cathodes for lithium-ion batteries. J Alloys Compd 810:151889
Wang B, Al Abdulla W, Wang D, Zhao XS (2015) A three-dimensional porous LiFePO4 cathode material modified with a nitrogen-doped graphene aerogel for high-power lithium ion batteries. Energy Environ Sci 8(3):869–875
Wu Y, Chong S, Chen Y (2023) Intermediate phase-assisted Li-intercalation/extraction behavior for LiFePO4 cathode materials. ACS Appl Energy Mater 6(18):9249–9255
Paolella A, Faure C, Bertoni G et al (2017) Light-assisted delithiation of lithium iron phosphate nanocrystals towards photo-rechargeable lithium ion batteries. Nat Commun 8(1):14643
Qiu N, Yang Z, Xue R et al (2021) Toward a high-performance aqueous zinc ion battery: potassium vanadate nanobelts and carbon enhanced zinc foil. Nano Lett 21(7):2738–2744
Han J-G, Lee JB, Cha A et al (2018) Unsymmetrical fluorinated malonatoborate as an amphoteric additive for high-energy-density lithium-ion batteries. Energy Environ Sci 11(6):1552–1562
Li B, Shao Y, He J et al (2022) Cyclability improvement of high voltage lithium cobalt oxide/graphite battery by use of lithium difluoro(oxalate)borate electrolyte additive. Electrochim Acta 426:140783
Li Y, Cheng B, Jiao F, Wu K (2020) The roles and working mechanism of salt-type additives on the performance of high-voltage lithium-ion batteries. ACS Appl Mater Interf 12(14):16298–16307
Cha J, Han J-G, Hwang J et al (2017) Mechanisms for electrochemical performance enhancement by the salt-type electrolyte additive, lithium difluoro(oxalato)borate, in high-voltage lithium-ion batteries. J Power Sources 357:97–106
Zheng H, Xiang H, Jiang F et al (2020) Lithium difluorophosphate-based dual-salt low concentration electrolytes for lithium metal batteries. Adv Energy Mater 10(30):2001440
Mao M, Huang B, Li Q et al (2020) In-situ construction of hierarchical cathode electrolyte interphase for high performance LiNi0.8Co0.1Mn0.1O2/Li metal battery. Nano Energy 78:105282
Yang T, Zeng H, Wang W et al (2019) Lithium bisoxalatodifluorophosphate (LiBODFP) as a multifunctional electrolyte additive for 5 V LiNi0.5Mn1.5O4-based lithium-ion batteries with enhanced electrochemical performance. J Mate Chem A 7(14):8292–8301
Li J, Xing L, Zhang R et al (2015) Tris(trimethylsilyl)borate as an electrolyte additive for improving interfacial stability of high voltage layered lithium-rich oxide cathode/carbonate-based electrolyte. J Power Sources 285:360–366
Wu F, Zhu Q, Chen R et al (2015) Ionic liquid electrolytes with protective lithium difluoro(oxalate)borate for high voltage lithium-ion batteries. Nano Energy 13:546–553
Li X, Lin Z, Jin N et al (2021) Perovskite-type SrVO3 as high-performance anode materials for lithium-ion batteries. Adv Mater 34(46):2107262
Li D, Ren X, Ai Q et al (2018) Facile fabrication of nitrogen-doped porous carbon as superior anode material for potassium-ion batteries. Adv Energy Mater 8(34):1802386
Funding
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (NSFC, 52101262), The Young and Middle-Aged Scientific and Technological Innovation Team in Colleges and Universities in Hubei Province (T2002005) and the 111 project (D20015), and the Open Research Fund Project of Hubei Key Laboratory of Natural Products Research and Development (China Three Gorges University, 2022NPRD05).
Author information
Authors and Affiliations
Contributions
Le Zhang: methodology, data curation, and writing—original draft. Pengju Li: methodology, data curation, conceptualization, supervision, and writing—review and editing. Dongmei Zhang: methodology and data curation. Cunyuan Pei: methodology and data curation. Bing Sun: methodology and data curation. Shibing Ni: project administration, funding acquisition, and supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Li, P., Zhang, D. et al. Lithium difluoro(oxalate)borate as an efficient and multifunctional additive for extended cycling life of LiFePO4 cathodes. Ionics 30, 2493–2501 (2024). https://doi.org/10.1007/s11581-024-05472-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05472-x