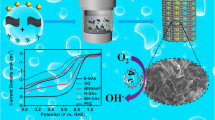
Abstract
Graphene oxide (GO) exhibits unique layered structures and excellent electrical conductivity, and the multiple surface termination groups are favorable for hosting impressive performance for electrochemical reactions. Therefore, a two-dimensional layered GO-based catalyst may become a novel high-efficiency electrocatalyst to replace traditional noble metal electrocatalysts. Herein, Co–Mo–B/GO (CMBG) nanocomposites are successfully prepared by electroless deposition for the oxygen evolution reaction (OER). GO constructs multi-layer three-dimensional structure with large surface area, which helps to increase electron transfer and mass transfer within the materials. Due to the introduction of Mo, the aggregation of nanoparticles on GO is reduced, and also the synergistic effect among Co, Mo, and B improves the electrocatalytic performance of as-prepared materials. Moreover, electrochemical tests reveal that the CMBG electrocatalyst can reduce the charge transfer resistance toward the OER and increase electrochemical active sites, leading to enhancing the OER performance. When the molar ratio of Mo/(Co + Mo) is 3:100, overpotential of CMBG is only 270 mV at 10 mA cm−2. In addition, CMBG shows long-term electrocatalytic stability even after 24-h test. This work provides a feasible way for the preparation of ternary boride electrocatalyst for OER.










Similar content being viewed by others
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
Wu Q, Li J, Wu T, Ji L, Zhang R, Jiang P, Chen H, Zhao R, Asiri A, Sun X (2019) One-step preparation of cobalt-nanoparticle-embedded carbon for effective water oxidation electrocatalysis. ChemElectroChem 6:1996–1999. https://doi.org/10.1002/celc.201900094
Li P, Zhao R, Chen H, Wang H, Wei P, Huang H, Liu Q, Li T, Shi X, Zhang Y, Liu M, Sun X (2019) Recent advances in the development of water oxidation electrocatalysts at mild pH. Small 15:1805103. https://doi.org/10.1002/smll.201805103
Pal D, Singh A, Bhatnagar A (2022) A review on biomass based hydrogen production technologies. Int J Hydrogen Energy 47:1461–1480. https://doi.org/10.1016/j.ijhydene.2021.10.1240360-3199
Lin L, Yu Q, Peng M, Li A, Yao S, Tian S, Liu X, Li A, Jiang Z, Gao R (2021) Atomically dispersed Ni/alpha-MoC catalyst for hydrogen production from methanol/water. J Am Chem Soc 143:309–317. https://doi.org/10.1021/jacs.0c10776
Wang X, Xiang R, Li S, Song K, Huang W (2023) Self-standing 2D/2D Co3O4@FeOOH nanosheet arrays as promising catalysts for the oxygen evolution reaction. Dalton Trans 52:2002–2012. https://doi.org/10.1039/d2dt03708d
Li Y, Wu Y, Hao H, Yuan M, Lv Z, Xu L, Wei B (2022) In situ unraveling surface reconstruction of Ni5P4@FeP nanosheet array for superior alkaline oxygen evolution reaction. Appl Catal B 305:121033. https://doi.org/10.1016/j.apcatb.2021.121033
Yu Z, Duan Y, Feng X, Yu X, Gao M, Yu S (2021) Clean and affordable hydrogen fuel from alkaline water splitting: past, recent progress, and future prospects. Adv Mater 33:2007100. https://doi.org/10.1002/adma.202007100
Suen N, Hung S, Quan Q, Zhang N, Xu Y, Chen H (2017) Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem Soc Rev 46:337–365. https://doi.org/10.1039/C6CS00328A
Song F, Bai L, Moysiadou A, Lee S, Hu C, Liardet L, Hu X (2018) Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J Am Chem Soc 140:7748–7759. https://doi.org/10.1021/jacs.8b04546
Lee Y, Suntivich J, May K, Perry E, Shao-Horn Y (2012) Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J Phys Chem Lett 3:399–404. https://doi.org/10.1021/jz2016507
Anand P, Zhou J, Tang T, Zhang G, Chen Y, Xu C (2020) A new generation of platinum-copper electrocatalysts with ultra-low concentrations of platinum for oxygen-reduction reactions in alkaline media. ChemistrySelect 5:3391–3397. https://doi.org/10.1002/slct.202000256
Xie X, Du L, Yon L, Park S, Qiu Y, Sokolowski J, Wang W, Shao Y (2022) Oxygen evolution reaction in alkaline environment: material challenges and solutions. Adv Funct Mater 32:2110036. https://doi.org/10.1002/adfm.202110036
He W, Wang F, Gao Y, Hao Q, Liu C (2022) One-step synthesis of amorphous transition metal sulfides as bifunctional electrocatalysts for the hydrogen evolution reaction and oxygen evolution reaction. Sustain Energy Fuels 6:3852–3857. https://doi.org/10.1039/d2se00886f
Li Y, Zhu S, Wu E, Ding H, Lu J, Mu X, Chen L, Zhang Y, Palisaitis J, Chen K, Li M, Yan P, Persson P, Hultman L, Eklund P, Du S, Kuang Y, Chai Z, Huang Q (2023) Nanolaminated ternary transition metal carbide (max phase)-derived core-shell structure electrocatalysts for hydrogen evolution and oxygen evolution reactions in alkaline electrolytes. J Phys Chem Lett 14:481–488. https://doi.org/10.1021/acs.jpclett.2c03230
Chen Q, Gong N, Zhu T, Yang C, Peng W, Li Y, Zhang F, Fan X (2021) Surface phase engineering modulated iron-nickel nitrides/alloy nanospheres with tailored d-band center for efficient oxygen evolution reaction. Small 18:2105696. https://doi.org/10.1002/smll.202105696
Wang L, Li J, Zhao X, Hao W, Ma X, Li S, Guo Y (2019) Surface-activated amorphous iron borides (FexB) as efficient electrocatalysts for oxygen evolution reaction. Adv Mater Interfaces 6:1801690. https://doi.org/10.1002/admi.201801690
Yue S, Wang S, Jiao Q, Feng X, Zhan K, Dai Y, Feng C, Li H, Feng T, Zhao Y (2020) Preparation of yolk-shell-structured CoxFe1-xP with enhanced OER performance. Chemsuschem 12:4461–4470. https://doi.org/10.1002/cssc.201901604
Liu T, Liu W, Ma M, Guo L, Cui R, Cheng D, Cao D (2023) Constructing nickel vanadium phosphide nanoarrays with highly active heterointerfaces for water oxidation in alkali media. Electrochim Acta 439:141721. https://doi.org/10.1016/j.electacta.2022.141721
Chunduri A, Gupta S, Bapat O, Bhide A, Fernandes R, Patel M, Bambole V, Miotello A, Patel N (2019) A unique amorphous cobalt-phosphide-boride bifunctional electrocatalyst for enhanced alkaline water splitting. Appl Catal B 259:118051. https://doi.org/10.1016/j.apcatb.2019.118051
Wang X, Zuo Y, Horta S, He R, Yang L, Moghaddam A, Ibáñez M, Qi X, Cabot A (2022) CoFeNiMnZnB as a high-entropy metal boride to boost the oxygen evolution reaction. ACS Appl Mater Interfaces 14:48212–48219. https://doi.org/10.1021/acsami.2c11627
Ding J, Zhu X, Yue R, Liu W, He S, Pei H, Zhu J, Zheng H, Liu N, Mo Z (2022) Ni-B-Co nanoparticles based on ZIF-67 as efficient electrocatalyst for oxygen evolution reaction. J Electroanal Chem 923:116838. https://doi.org/10.1016/j.jelechem.2022.116838
Tian L, Zhong D, Zhao T, Liu Y, Hao L, Fang Q, Lang X, Zhao X, Hao G, Liu G, Li J, Zhao Q (2023) Oxygen-vacancy-rich Co3O4@Fe-B-O heterostructure for efficient oxygen evolution reaction in alkaline and neutral media. J Colloid Interface Sci 646:452–460. https://doi.org/10.1016/j.jcis.2023.05.042
Patil K, Babar P, Li X, Karade V, Kim S, Jang S, Bhoite P, Kim J (2022) Co-Fe-B nanochain electrocatalysts for oxygen evolution at high current density. ACS Appl Nano Mater 5:6260–6267. https://doi.org/10.1021/acsanm.2c00312
Yang P, Li E, Xiao F, Zhou P, Wang Y, Tang W, He P, Jia B (2022) Nanostructure Fe-Co-B/bacterial cellulose based carbon nanofibers: an extremely efficient electrocatalyst toward oxygen evolution reaction. Int J Hydrogen Energy 47:12953–12963. https://doi.org/10.1016/j.ijhydene.2022.02.0530360-3199
Li P, Jin Z, Xiao D (2014) A one-step synthesis of Co-P-B/rGO at room temperature with synergistically enhanced electrocatalytic activity in neutral solution. J Mater Chem A 2:18420–18427. https://doi.org/10.1039/c4ta03962a
Tripathy R, Samantara A, Behera J (2021) Metal-organic framework (MOF)-derived amorphous nickel boride: an electroactive material for electrochemical energy conversion and storage application. Sustain Energy Fuels 5:1184–1193. https://doi.org/10.1039/d0se01831g
Lei W, Jin H, Gao J, Chen Y (2021) Efficient hydrogen generation from the NaBH4 hydrolysis by amorphous Co–Mo–B alloy supported on reduced graphene oxide. J Mater Res 36:4154–4168. https://doi.org/10.1557/s43578-021-00374-4
Li Y, Xu H, Huang H, Gao L, Zhao Y, Ma T (2018) Synthesis of Co-B in porous carbon using a metal-organic framework (MOF) precursor: a highly efficient catalyst for the oxygen evolution reaction. Electrochem Commun 86:140–144. https://doi.org/10.1016/j.elecom.2017.12.011
Wang X, He Q, Ge Q, Fu T, Chen S, Zhou P, Xiao F, He P, Jia L, Jia B, Zhang H, Pan K, Liu H (2021) Self-assembled nanocotton-like Co-B-P/bacterial cellulose based carbon nanofiber as highly efficient electrocatalyst for oxygen evolution reaction. Int J Hydrogen Energy 46:20930–20940. https://doi.org/10.1016/j.ijhydene.2021.03.211
Elumeeva K, Masa J, Medina D, Ventosa E, Seisel S, Kayran Y, Genc A, Bobrowski T, Weide P, Arbiol J, Muhler M, Schuhmann W (2017) Cobalt boride modified with N-doped carbon nanotubes as a high-performance bifunctional oxygen electrocatalyst. J Mater Chem A 5:21122–21129. https://doi.org/10.1039/c7ta06995b
Sekar S, Ahmed A, Pawar S, Lee Y, Im H, Kim D, Lee S (2020) Enhanced water splitting performance of biomass activated carbon-anchored WO3 nanoflakes. Appl Surf Sci 508:145127. https://doi.org/10.1016/j.apsusc.2019.145127
Singh S, Nguyen D, Kim N, Lee J (2022) Interface engineering induced electrocatalytic behavior in core-shelled CNTs@NiP2/NbP heterostructure for highly efficient overall water splitting. Chem Eng J 442:136120. https://doi.org/10.1016/j.cej.2022.136120
Sun L, Xu H, Yang Y, Li L, Zhao X, Zhang W (2023) Graphitic carbon-encapsulated cobalt nanoparticles embedded 1D porous hollow carbon nanofibers as advanced multifunctional electrocatalysts for overall water splitting and Zn-air batteries. Int J Hydrogen Energy 48:5095–5106. https://doi.org/10.1016/j.ijhydene.2022.11.058
Li Q, Sun Z, Yin C, Chen Y, Pan D, Yu B, Zhang Y, He T, Chen S (2023) Template-assisted synthesis of ultrathin graphene aerogels as bifunctional oxygen electrocatalysts for water splitting and alkaline/neutral zinc-air batteries. Chem Eng J 458:141492. https://doi.org/10.1016/j.cej.2023.141492
Roy O, Jana A, Pratihar B, Saha D, De S (2022) Graphene oxide wrapped Mix-valent cobalt phosphate hollow nanotubes as oxygen evolution catalyst with low overpotential. J Colloid Interface Sci 610:592–600. https://doi.org/10.1016/j.jcis.2021.11.112
Zhuang W, Li Z, Song M, Zhu W, Tian L (2022) Synergistic improvement in electron transport and active sites exposure over rGO supported NiP/Fe4P for oxygen evolution reaction. Ionics 28:1359–1366. https://doi.org/10.1007/s11581-021-04396-0
He C, Hu X, Peng X, Zhao Y, Li Y, Li X, Fan L, Zhang Y (2023) In situ facile fabrication of ultrathin Co(OH)2-CoO/graphene oxide nanosheet hybrids with superior oxygen evolution reaction performance. J Alloys Compd 948:169780. https://doi.org/10.1016/j.jallcom.2023.169780
Sun Y, Wang Q, Geng Z, Liu Z, Yang R (2021) Fabrication of two-dimensional 3d transition metal oxides through template assisted cations hydrolysis method. Chem Eng J 415:129044. https://doi.org/10.1016/j.cej.2021.129044
Ensafi A, Haghighi M, Jafari-Asl M (2018) Phosphine-functionalized graphene oxide, a high-performance electrocatalyst for oxygen reduction reaction. Appl Surf Sci 427:722–729. https://doi.org/10.1016/j.apsusc.2017.08.011
Yang H, Guo T, Yin D, Liu Q, Zhang X, Zhang X (2020) A high-efficiency noble metal-free electrocatalyst of cobalt-iron layer double hydroxides nanorods coupled with graphene oxides grown on a nickel foam towards methanol electrooxidation. J Taiwan Inst Chem Eng 112:212–221. https://doi.org/10.1016/j.jtice.2020.06.012
Nagajyothi P, Pavani K, Ramaraghavulu R, Shim J (2023) Ce-metal-organic framework-derived CeO2-GO: an efficient electrocatalyst for oxygen evolution reaction. Inorganics 11:161. https://doi.org/10.3390/inorganics11040161
Zhao W, Liu T, Wu N, Zhou B, Yan Y, Ye Y, Gong J, Yang S (2022) Bimetallic electron-induced phase transformation of CoNi LDH-GO for high oxygen evolution and supercapacitor performance. Sci China Mater 66:577–586. https://doi.org/10.1007/s40843-022-2170-6
Gao J, He P, Yang T, Wang X, Zhou L, He Q, Jia L, Deng H, Zhang H, Jia B (2020) Short rod-like Ni-MOF anchored on graphene oxide nanosheets: a promising voltammetric platform for highly sensitive determination of pchloronitrobenzene. J Electroanal Chem 861:113954. https://doi.org/10.1016/j.jelechem.2020.113954
Wang S, He P, Xie Z, Jia L, He M, Zhang X, Dong F, Liu H, Zhang Y, Li C (2019) Tunable nanocotton-like amorphous ternary Ni-Co-B: a highly efficient catalyst for enhanced oxygen evolution reaction. Electrochim Acta 296:644–652. https://doi.org/10.1016/j.electacta.2018.11.099
Mo M, Zheng M, Tang J, Lu Q, Xun Y (2014) Highly active Co-B, Co-Mo(W)-B amorphous nanotube catalysts for the selective hydrogenation of cinnamaldehyde. J Mater Sci 49:877–885. https://doi.org/10.1007/s10853-013-7771-1
Xu J, Wang K, Zu S, Han B, Wei Z (2010) Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 4:5019–5026. https://doi.org/10.1021/nn1006539
Ramesha G, Kumara A, Muralidhara H, Sampath S (2011) Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J Colloid Interface Sci 361:270–277. https://doi.org/10.1016/j.jcis.2011.05.050
Yuan H, Liu J, Jiao Q, Li Y, Liu X, Shi D, Wu Q, Zhao Y, Li H (2017) Sandwich-like octahedral cobalt disulfide/reduced graphene oxide as an efficient Pt-free electrocatalyst for high-performance dye-sensitized solar cells. Carbon 119:225–234. https://doi.org/10.1016/j.carbon.2017.04.040
Yan S, He P, Jia D, Yang Z, Duan X, Wang S, Zhou Y (2016) Effects of treatment temperature on the reduction of GO under alkaline solution during the preparation of graphene/geopolymer composites. Ceram Int 42:18181–18188. https://doi.org/10.1016/j.ceramint.2016.08.134
Zainab S, Azeem M, Awan S, Rizwan S, Iqbal N, Rashid J (2023) Optimization of bandgap reduction in 2-dimensional GO nanosheets and nanocomposites of GO/iron-oxide for electronic device applications. Sci Rep 13:6954. https://doi.org/10.1038/s41598-023-33200-4
Wu C, Li J (2017) Unique hierarchical Mo2C/C nanosheet hybrids as active electrocatalyst for hydrogen evolution reaction. ACS Appl Mater Interfaces 9:41314–41322. https://doi.org/10.1021/acsami.7b13822
Zakrzewska B, Jablonska A, Adamczyk L, Dembinska B, Kostuch A, Strawski M, Rutkowska I, Kulesza P, Marcinek M, Cox J, Miecznikowski K (2023) Pyrolyzed cobalt hexacyanocobaltate dispersed on reduced-graphene-oxide as an electrocatalyst of the oxygen reduction reaction in an alkaline medium. J Mater Chem A 11:7286–7298. https://doi.org/10.1039/d2ta08682d
Zhang X, Chen Y, Wang B, Chen M, Yu B, Wang X, Zhang W, Yang D (2019) FeNi nanoparticles embedded porous nitrogen-doped nanocarbon as efficient electrocatalyst for oxygen evolution reaction. Electrochim Acta 321:134720. https://doi.org/10.1016/j.electacta.2019.134720
Chen N, Mo Q, He L, Huang X, Yang L, Zeng J, Gao Q (2019) Heterostructured MoC-MoP/N-doped carbon nanofibers as efficient electrocatalysts for hydrogen evolution reaction. Electrochim Acta 299:708–716. https://doi.org/10.1016/j.electacta.2019.01.054
Shi Y, Hu X, Zhu B, Zhang S, Huang W (2015) Hydroformylation of 1-octene over nanotubular TiO2-supported amorphous Co-B catalysts. Chem Res Chin Univ 31:851–857. https://doi.org/10.1007/s40242-015-5002-9
Liu H, He P, Wang S, Gao J, Zhou L, Li C, Zhang Y, Yang D, He M, Jia L, Dong F, Liu H (2019) Facile one-step fabrication of bimetallic Co-Ni-P hollow nanospheres anchored on reduced graphene oxide as highly efficient electrocatalyst for hydrogen evolution reaction. Int J Hydrogen Energy 44:24140–24150. https://doi.org/10.1016/j.ijhydene.2019.07.139
Cao X, Wang X, Cui L, Jiang D, Zheng Y, Liu J (2017) Strongly coupled nickel boride/graphene hybrid as a novel electrode material for supercapacitors. Chem Eng J 327:1085–1092. https://doi.org/10.1016/j.cej.2017.07.010
Zhou L, Yang T, Chen S, Gao J, Wang X, He P, Lei H, Yang D, Dong F, Jia L, Jia B, Zhang H (2020) Tunably fabricated nanotremella-like Bi2S3/MoS2: an excellent and highly stable electrocatalyst for alkaline hydrogen evolution reaction. Int J Hydrogen Energy 45:9535–9545. https://doi.org/10.1016/j.ijhydene.2020.01.168
Wang T, Wang P, Zang W, Li X, Chen D, Kou Z, Mu S, Wang J (2021) Nanoframes of Co3O4-Mo2N heterointerfaces enable high-performance bifunctionality toward both electrocatalytic HER and OER. Adv Funct Mater 32:2107382. https://doi.org/10.1002/adfm.202107382
Yan J, Liu P, Li J, Huang H, Song W (2023) Effect of valence state on electrochemical nitrate reduction to ammonia in molybdenum catalysts. Chem Eng J 459:141601. https://doi.org/10.1016/j.cej.2023.141601
Peng G, Zhao J, Wang J, Hoenig E, Wu S, Wang M, He M, Zhang L, Liu J, Liu C (2023) Crystal structures of molybdenum borides dictate electrocatalytic ammonia synthesis efficiency. Appl Catal B 338:123020. https://doi.org/10.1016/j.apcatb.2023.123020
Zhou Y, Zhang J, Ren H, Pan Y, Yan Y, Sun F, Wang X, Wang S, Zhang J (2020) Mo doping induced metallic CoSe for enhanced electrocatalytic hydrogen evolution. Appl Catal B 268:118467. https://doi.org/10.1016/j.apcatb.2019.118467
Liu Y, Luo X, Zhou C, Du S, Zhen D, Chen B, Li J, Wu Q, Iru Y, Chen D (2020) A modulated electronic state strategy designed to integrate active HER and OER components as hybrid heterostructures for efficient overall water splitting. Appl Catal B 260:118197. https://doi.org/10.1016/j.apcatb.2019.118197
Shan H, Qin J, Ding Y, Sari H, Song X, Liu W, Hao Y, Wang J, Xie C, Zhang J, Li X (2021) Controllable heterojunctions with a semicoherent phase boundary boosting the potassium storage of CoSe2/FeSe2. Adv Mater 33:2102471. https://doi.org/10.1002/adma.202102471
Liu P, Yang S, Zheng L, Zhang B, Yang H (2017) Mo6+ activated multimetal oxygen-evolving catalyst. Chem Sci 8:3484–3488. https://doi.org/10.1039/c6sc04819f
Wang C, Wang Q, Wang K, De Ras M, Chu K, Gu L, Lai F, Qiu S, Guo H, Zuo P, Hofkens J, Zhu X (2023) Bimetallic NiCo boride nanoparticles confined in a MXene network enable efficient ambient ammonia electrosynthesis. J Energy Chem 77:469–478. https://doi.org/10.1016/j.jechem.2022.11.010
Chen R, Liu L, Zhou J, Hou L, Gao F (2017) High-performance nickel-cobalt-boron material for an asymmetric supercapacitor with an ultrahigh energy density. J Power Sources 341:75–82. https://doi.org/10.1016/j.jpowsour.2016.11.108
Wu P, Wu C, Chen D (2018) Synthesis and controlled sulfidation of Ni-Co alloy on reduced graphene oxide as an electrode with enhanced conductivity and capacitance for supercapacitors. J Alloys Compd 735:409–416. https://doi.org/10.1016/j.jallcom.2017.11.041
Karuppasamy K, Bose R, Vikraman D, Ramesh S, Kim H, Alhseinat E, Alfantazi A, Kim H (2023) Revealing the effect of various organic ligands on the OER activity of MOF-derived 3D hierarchical cobalt oxide @ carbon nanostructures. J Alloys Compd 934:167909. https://doi.org/10.1016/j.jallcom.2022.167909
Vikraman D, Hussain S, Abbas Z, Karuppasamy K, Santhoshkumar P, Jung J, Kim H (2023) Density functional theory approximations and experimental investigations on Co1-xMoxTe2 alloy electrocatalysts tuning the overall water splitting reactions. ACS Appl Mater Interfaces 15:26893–26909. https://doi.org/10.1021/acsami.3c05055
Liu H, Zeng S, He P, Dong F, He M, Zhang Y, Wang S, Li C, Liu M, Jia L (2019) Samarium oxide modified Ni-Co nanosheets based three-dimensional honeycomb film on nickel foam: a highly efficient electrocatalyst for hydrogen evolution reaction. Electrochim Acta 299:405–414. https://doi.org/10.1016/j.electacta.2018.12.169
He C, Bo T, Wang B, Tao J (2019) RGO induced one-dimensional bimetallic carbide nanorods: an efficient and pH-universal hydrogen evolution reaction electrocatalyst. Nano Energy 62:85–93. https://doi.org/10.1016/j.nanoen.2019.05.009
Vikraman D, Hussain S, Hailiang L, Karuppasamy K, Sivakumar P, Santhoshkumar P, Jung J, Kim H (2022) Spinel-structured metal oxide-embedded MXene nanocomposites for efficient water splitting reactions. Inorg Chem Front 9:5903–5916. https://doi.org/10.1039/d2qi01564a
Cao J, Zhang Y, Liu X, Zhang C, Li Z (2022) Comparison of Co-Mo-S and remote control model for designing efficient Co-doped MoS2 hydrodeoxygenation catalysts. Fuel 334:126640. https://doi.org/10.1016/j.fuel.2022.126640
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 51602267), the Major Science and Technology Project of Sichuan Province (Grant No. 2019YFS0090, 2022YFS0614), and the International Science and Technology Cooperation Laboratory of Micro-nanoparticle Application Research of Southwest University of Science and Technology (Grant No. 19MNA001).
Author information
Authors and Affiliations
Contributions
Qihang He contributed to investigation; writing, original draft; and writing, review and editing. Lei Wang contributed to investigation; writing, original draft; and writing, review and editing. Feng Xiao contributed to investigation and writing, review and editing. Rong Su contributed to investigation and writing, review and editing. Lichuan Chen contributed to investigation and writing, review and editing. Yu Jiang contributed to investigation and writing, review and editing. Bin Jia contributed to project administration and formal analysis. Ping He contributed to project administration and writing, review and editing. Yali Zeng contributed to project administration and formal analysis. Yun Zhou contributed to project administration and writing, review and editing. Ying Wan contributed to project administration and formal analysis. Bin Tang contributed to project administration and writing, review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Q., Wang, L., Xiao, F. et al. Strongly coupled cobalt–molybdenum–boron nanoparticles anchored on graphene oxide as highly efficient electrocatalyst for oxygen evolution reaction. Ionics 30, 2259–2271 (2024). https://doi.org/10.1007/s11581-024-05443-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05443-2