Abstract
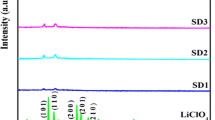
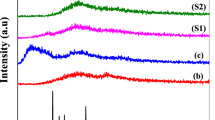
In this work, a comparative study is presented utilizing a K+ conducting gel polymer electrolyte (GPE) system comprising of a poly(vinylidene fluoride-hexafluoropropylene) (PVdF-HFP) polymer matrix combined with potassium permanganate (KMnO4) salt and two different plasticizers as a binary combination of ethylene carbonate (EC) + propylene carbonate (PC) and tetraethylene glycol dimethyl ether (TEGDME). The electrolyte with TEGDME solvent shows an ionic conductivity (σ) of 7.01 × 10−6 S cm−1 with hopping of ions as charge transport behavior. The fabricated electrolyte possesses > 98% contribution from the ions, and the electro-chemical stability range is up to 3.2 V. The dielectric analysis shows that the electrolyte with TEGDME solvent possesses a higher value of the dielectric constant, dielectric loss, tangent loss, and dc conductivity, along with a higher free ion number density (N) in comparison to the counter-electrolyte specimens with EC:PC solvent. The thermal studies confirm the gel phase of both electrolytes with negligible weight loss till 100 °C. Polymer-salt complex interactions are confirmed using Fourier transform infrared spectroscopy (FTIR), while X-ray diffraction (XRD) analysis is used to study the variations in crystallinity brought about by the polymer and both electrolyte samples.








Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Patel M, Mishra K, Banerjee R, Chaudhari J, Kanchan DK, Kumar D (2023) Fundamentals, recent developments and prospects of lithium and non-lithium electrochemical rechargeable battery systems. J Energy Chem 81:221–259. https://doi.org/10.1016/j.jechem.2023.02.023
Kumar D, Gohel K, Kanchan DK, Mishra K (2020) Dielectrics and battery studies on flexible nanocomposite gel polymer electrolyte membranes for sodium batteries. J Mater Sci Mater Electron 31:13249–13260. https://doi.org/10.1007/s10854-020-03877-8
Kumar V A, John B, TD M (2020) Potassium ion batteries: key to future large scale energy storage? Appl Energy Mater 3:9478–9492. https://doi.org/10.1021/acsaem.0c01574
Ravi M, Bhavani S, Pavani Y, Narasimha Rao VVR (2013) Investigation on electrical and dielectric properties of PVP:KCLO4 polymer electrolyte films. Indian J Pure Appl Phys 51:362–366. https://doi.org/10.1016/j.solidstatesciences.2013.02.006
Patel M, Singh R, Prajapati AK, Kumar Y, Hmar JJL, Chaki SH, Kanchan DK, Kumar D (2023) Effect of varying lithium perchlorate salt concentration on electrochemical and physical properties of polymer gel electrolytes containing heat-resistant poly(methyl methacrylate) and succinonitrile. J Appl Electrochem. https://doi.org/10.1007/s10800-023-02014-7
Syali MS, Kumar D, Mishra K, Kanchan DK (2020) Recent advances in electrolytes for room-temperature sodium-sulfur batteries: a review. Energy Stor Mater 31:352–372. https://doi.org/10.1016/j.ensm.2020.06.023
Ahmad AL, Farooqui UR, Hamid NA (2018) Effect of graphene oxide (GO) on poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) polymer electrolyte membrane. Polymer (Guildf) 142:330–336. https://doi.org/10.1016/j.polymer.2018.03.052
Shalu S, Singh VK, Singh RK (2015) Development of ion conducting polymer gel electrolyte membranes based on polymer PVdF-HFP, BMIMTFSI ionic liquid and the Li-salt with improved electrical, thermal and structural properties. J Mater Chem C Mater 3:7305–7318. https://doi.org/10.1039/C5TC00940E
Bhide A, Hariharan K (2007) Ionic transport studies on (PEO)6:NaPO3 polymer electrolyte plasticized with PEG400. Eur Polym J 43:4253–4270. https://doi.org/10.1016/j.eurpolymj.2007.07.038
Dave G, Maheshwaran C, Kanchan D (2019) Conductivity enhancement in Na+-blend electrolyte system via addition of (EC+PC) plasticizers. AIP Conf Proc 2115:030234. https://doi.org/10.1063/1.5113073
Razalli SMM, Saaid SIYSM, Kudin TIT, Yahya MZA, Hassan OH, Ali MM (2016) Electrochemical properties of glyme based plasticizer on gel polymer electrolytes doped with lithium bis(trifluoromethanesulfonyl)imide. Mater Sci Forum 846:534–538. https://doi.org/10.4028/www.scientific.net/MSF.846.534
Singh R, Maheshwaran C, Kanchan DK, Mishra K, Singh PK, Kumar D (2021) Ion-transport behavior in tetraethylene glycol dimethyl ether incorporated sodium ion conducting polymer gel electrolyte membranes intended for sodium battery application. J Mol Liq 336:116594. https://doi.org/10.1016/j.molliq.2021.116594
Yadav N, Hashmi SA (2020) Energy enhancement of quasi-solid-state supercapacitors based on a non-aqueous gel polymer electrolyte via a synergistic effect of dual redox additives diphenylamine and potassium iodide. J Mater Chem A Mater 8:18266–18279. https://doi.org/10.1039/D0TA06331B
Yin H, Han C, Liu Q, Wu F, Zhang F, Tang Y (2021) Recent advances and perspectives on the polymer electrolytes for sodium/potassium-ion batteries Small:2006627. https://doi.org/10.1002/smll.202006627
Ngai KS, Ramesh S, Ramesh K, Juan JC (2016) A review of polymer electrolytes: fundamental, approaches and applications. Ionics 22:1259–1279. https://doi.org/10.1007/s11581-016-1756-4
Madhani V, Rathore MS, Kumar D (2023) The effects of solvents on the physical and electrochemical properties of potassium-ion conducting polymer gel electrolytes. High Perform Polym 35:28–35. https://doi.org/10.1177/09540083221112310
Ford HO, Cui C, Schaefer JL (2020) Comparison of single-ion conducting polymer gel electrolytes for sodium, potassium, and calcium batteries: influence of polymer chemistry, cation identity, charge density, and solvent on conductivity. Batteries 6. https://doi.org/10.3390/batteries6010011
Jyothi NK, Venkataratnam KK, Murty PN, Kumar KV (2016) Preparation and characterization of PAN–KI complexed gel polymer electrolytes for solid-state battery applications. Bull Mater Sci 39:1047–1055. https://doi.org/10.1007/s12034-016-1241-8
Jibreel UM, Shehu AA, Ahmad AA, Adiya ZSG, Salisu S (2021) Investigation, synthesis, characterization and detail studies of polymer electrolyte films of poly (ethylmethacrylate) with potassium iodide as doping salt and ethylene carbonate as plasticizer (PEMA: KI + EC). Am J Sci Eng Res 5:24–33. https://iarjournals.com/upload/452433.pdf. Accessed 2 Sept 2021
Hor AA, Yadav N, Hashmi SA (2022) High energy density carbon supercapacitor with ionic liquid-based gel polymer electrolyte: role of redox-additive potassium iodide. J Energy Storage 47:103608. https://doi.org/10.1016/j.est.2021.103608
Babu B, Neumann C, Muench S, Enke M, Medenbach L, Leibing C, Balducci A, Turchanin A, Schubert US, Balducci A (2023) Diglyme-based gel polymer electrolytes for K-ion capacitors. Energy Stor Mater 56:342–350. https://doi.org/10.1016/j.ensm.2023.01.031
Ravindran D, Vickraman P (2012) XRD Conductivity studies on PVA-PEG blend based Mg2+ ion conducting polymer electrolytes. Int J Sci Eng Appl:72–74. https://doi.org/10.7753/ijsea0101.1012
Ahmed HT, Abdullah OGh (2020) Structural and ionic conductivity characterization of PEO:MC-NH4I proton-conducting polymer blend electrolytes based films. Results Phys 16:102861. https://doi.org/10.1016/j.rinp.2019.102861
Abdullah OGh, Ahmed HT, Tahir DA, Jamal GM, Mohamad AH (2021) Influence of PEG plasticizer content on the proton-conducting PEO:MC-NH4I blend polymer electrolytes based films. Results Phys 23:104073. https://doi.org/10.1016/j.rinp.2021.104073
Tong Y, Que M, Su S, Chen L (2016) Design of amphiphilic poly(vinylidene fluoride-co- hexafluoropropylene)-based gel electrolytes for high-performance lithium-ion batteries. Ionics (Kiel) 22:1311–1318. https://doi.org/10.1007/s11581-016-1662-9
Zainuddin Z, Hambali D, Supa’at I, Osman Z (2017) Ionic conductivity, ionic transport and electrochemical characterizations of plastic crystal polymer electrolytes. Ionics (Kiel) 23:265–273. https://doi.org/10.1007/s11581-016-1836-5. 27 Sept 2016
Abdullah OGh, Hanna RR, Ahmed HT, Mohamad AH, Saleem SA, Saeed MAM (2021) Conductivity and dielectric properties of lithium-ion biopolymer blend electrolyte based film. Results Phys 24:104135. https://doi.org/10.1016/j.rinp.2021.104135
Rathika R, Suthanthiraraj SA (2019) Effect of ionic liquid 1-ethyl-3-methylimidazolium hydrogen sulfate on zinc-ion dynamics in PEO/PVdF blend gel polymer electrolytes. Ionics (Kiel) 25:1137–1146. https://doi.org/10.1007/s11581-018-2709-x
Ahmed MB et al (2022) The study of ion transport parameters associated with dissociated cation using EIS model in solid polymer electrolytes (SPEs) based on PVA host polymer: XRD, FTIR, and dielectric properties. Arab J Chem 15:104196. https://doi.org/10.1016/j.arabjc.2022.104196
Asnawi ASFM, Aziz SB, Nofal MM, Hamsan MH, Brza MA, Yusof YM, Abdilwahid RT, Muzakir SK, Kadir MFZ (2020) Glycerolized Li+ Ion conducting chitosan-based polymer electrolyte for energy storage EDLC device applications with relatively high energy density. Polymers (Basel) 12:1433. https://doi.org/10.3390/polym12061433
Sugumaran T, Silvaraj DS, Saidi NM, Farhana NK, Ramesh S, Ramesh K, Ramesh S (2019) The conductivity and dielectric studies of polymer electrolytes based on iota-carrageenan with sodium iodide and 1-butyl-3-methylimidazolium iodide for the dye- sensitized solar cells. Ionics (Kiel) 25:763–771. https://doi.org/10.1007/s11581-018-2756-3
Zulkifli AM, Said NIA, Aziz SB, Dannoun EM, Hisham S, Shah S, Bakar A, Zainal Z, Tajuddin HA, Hadi JM, Brza MA, Saeed SR, Amin PO (2020) Characteristics of dye-sensitized solar cell assembled from modified chitosan-based gel polymer electrolytes incorporated with potassium iodide. Molecules 25:4115. https://doi.org/10.3390/molecules25184115
Singh R, Singh PK, Singh V, Bhattacharya B (2016) Agarose biopolymer electrolytes: ion conduction mechanism and dielectric studies. Cellul Chem Technol 51:949–955
Singh D, Kanjilal D, Laxmi G, Singh PK, Tomar S, Bhattacharya B (2018) Conductivity and dielectric studies of Li 3+-irradiated PVP-based polymer electrolytes. High Perform Polym 30:978–985. https://doi.org/10.1177/0954008318780494
Abdulkadir BA, Dennis JO, Shukur MFBA, Nasef MME, Usman F (2021) Study on dielectric properties of gel polymer electrolyte based on PVA-K2CO3 composites. Int J Electrochem Sci 16:150296. https://doi.org/10.20964/2021.01.34
Sravanthi K, Sundari GS (2022) Optical and electric modulus studies of PMMA: CH3COOLi complexes with the addition of EMIMTFSI. Rasayan J Chem 15:2310–2317. https://doi.org/10.31788/RJC.2022.1547055
Bhargav PB, Mohan VM, Sharma AK, Rao VVRN (2009) Investigations on electrical properties of (PVA:NaF) polymer electrolytes for electrochemical cell applications. Curr Appl Phys 9:165–171. https://doi.org/10.1016/j.cap.2008.01.006
Verma ML, Sahu HD (2017) Study on ionic conductivity and dielectric properties of PEO- based solid nanocomposite polymer electrolytes. Ionics (Kiel) 23:2339–2350. https://doi.org/10.1007/s11581-017-2063-4
Arya A, Sharma AL (2019) Temperature and salt-dependent dielectric properties of blend solid polymer electrolyte complexed with LiBOB. Macromol Res 27:334–345. https://doi.org/10.1007/s13233-019-7077-5
Singh C, Shukla P, Agrawal S (2020) Ion transport studies in PVA:NH 4 CH 3 COO gel polymer electrolytes. High Perform Polym 32:208–219. https://doi.org/10.1177/0954008319898242
Singh P, Gupta PN, Saroj AL (2020) Ion dynamics and dielectric relaxation behavior of PVA- PVP-NaI-SiO2 based nano-composites polymer blend electrolytes. Physica B Condens Matter 578:411850. https://doi.org/10.1016/j.physb.2019.411850
Gohel K, Kanchan DK, Machhi HK, Soni SS, Maheshwaran C (2020) Gel polymer electrolyte based on PVDF-HFP:PMMA incorporated with propylene carbonate (PC) and diethyl carbonate (DEC) plasticizers: electrical, morphology, structural and electrochemical properties. Mater Res Express 7:025301. https://doi.org/10.1088/2053-1591/ab6c06
Tuhania P, Singh PK, Bhattacharya B, Dhapola PS, Yadav S, Shukla PK, Gupta M (2018) PVDF-HFP and 1-ethyl-3-methylimidazolium thiocyanate–doped polymer electrolyte for efficient supercapacitors. High Perform Polym 30:911–917. https://doi.org/10.1177/0954008318772009
Bandaranayake CM, Weerasinghe WADSS, Vidanapathirana KP, Perera KS (2016) A cyclic voltammetry study of a gel polymer electrolyte based redox-capacitor. Sri Lankan J Phys 16:19. https://doi.org/10.4038/sljp.v16i1.8026
Manfo T A, Konwar S, Singh PK, Mehra RM, Kumar Y, Gupta M (2021) PEO + NaSCN and ionic liquid based polymer electrolyte for supercapacitor. Mater Today Proc 34:802–812. https://doi.org/10.1016/j.matpr.2020.05.340
Jothi MA, Vanitha D, Bahadur SA, Nallamuthu N (2021) Proton conducting polymer electrolyte based on cornstarch, PVP, and NH4Br for energy storage applications. Ionics (Kiel) 27:225–237. https://doi.org/10.1007/s11581-020-03792-2
Hamsan MH, Shukur MF, Kadir MFZ (2017) The effect of NH4NO3 towards the conductivity enhancement and electrical behavior in methyl cellulose-starch blend based ionic conductors. Ionics (Kiel) 23:1137–1154. https://doi.org/10.1007/s11581-016-1918-4
Wilson J, Ravi G, Kulandainathan MA (2006) Electrochemical studies on inert filler incorporated poly (vinylidene fluoride - hexafluoropropylene) (PVdF - HFP) composite electrolytes. Polímeros 16:88–93. https://doi.org/10.1590/S0104-14282006000200006
Tripathi M, Bobade SM, Kumar A (2019) Preparation of polyvinylidene fluoride-co- hexafluoropropylene-based polymer gel electrolyte and its performance evaluation for application in EDLCs. Bull Mater Sci 42:27. https://doi.org/10.1007/s12034-018-1685-0
Aziz SB, Marf AS, Dannoun EMA, Brza MA, Abdullah RM (2020) The study of the degree of crystallinity, electrical equivalent circuit, and dielectric properties of polyvinyl alcohol (PVA)-based biopolymer electrolytes. Polymers (Basel) 12:2184. https://doi.org/10.3390/polym12102184
Hamsan MH, Shukur MF, Kadir MFZ (2017) NH4NO3 as charge carrier contributor in glycerolized potato starch-methyl cellulose blend-based polymer electrolyte and the application in electrochemical double-layer capacitor. Ionics (Kiel) 23:3429–3453. https://doi.org/10.1007/s11581-017-2155-1
Tripathi M, Kumar A (2018) Zinc oxide nanofiller-based composite polymer gel electrolyte for application in EDLCs. Ionics (Kiel) 24:3155–3165. https://doi.org/10.1007/s11581-018-2504-8
Muthupradeepa R, Sivakumar M, Subadevi R, Ramachandran M, Palanisamy R, Yuvakkumar R (2020) Physical and electrochemical chattels of phosphonium ionic liquid- based solid and gel-polymer electrolyte for lithium secondary batteries. J Mater Sci Mater Electron 31:22933–22944. https://doi.org/10.1007/s10854-020-04820-7
Mahendrakar S, Anna M, Reddy M J (2015) Structural, morphological and FTIR of PVDF-HFP and lithium tetrafluoroborate salt as polymer electrolyte membrane in lithium ion batteries. Int J Chemtech Res 8:319–328
Gohel K, Kanchan DK (2018) Ionic conductivity and relaxation studies in PVDF-HFP:PMMA-based gel polymer blend electrolyte with LiClO 4 salt. J Adv Dielectr 08:1850005. https://doi.org/10.1142/S2010135X18500054
Arya A, Sharma AL (2018) Optimization of salt concentration and explanation of two peak percolation in blend solid polymer nanocomposite films. J Solid State Electrochem 22:2725–2745. https://doi.org/10.1007/s10008-018-3965-4
Singh SK, Gupta H, Balo L, Na S, Singh VK, Tripathi AK, Verma YL, Singh RK (2018) Electrochemical characterization of ionic liquid based gel polymer electrolyte for lithium battery application. Ionics (Kiel) 24:1895–1906. https://doi.org/10.1007/s11581-018-2458-x
Arya A, Sharma AL (2020) Investigation on enhancement of electrical, dielectric and ion transport properties of nanoclay-based blend polymer nanocomposites. Polym Bull 77:2965–2999. https://doi.org/10.1007/s00289-019-02893-x
Gupta A, Jain A, Tripathi SK (2021) Structural, electrical and electrochemical studies of ionic liquid‑based polymer gel electrolyte using magnesium salt for supercapacitor application. J Polym Res 28:235. https://doi.org/10.1007/s10965-021-02597-9
Acknowledgements
Vaishali Madhani acknowledges the DST-FIST program of Applied Physics Department (SR/FST/PS-II/2017/20) of The M.S. University of Baroda for XRD characterization of samples. Vaishali Madhani and Mahendrasingh Rathore are grateful to the Parul University for their unending research support. Deepak Kumar acknowledges Science and Engineering Research Board, Department of Science and Technology, Government of India vide file No. CRG/2022/008719.
Funding
Research project by Science and Engineering Research Board, Department of Science and Technology, Government of India vide file No. CRG/2022/008719.
Author information
Authors and Affiliations
Contributions
Vaishali Madhani: Sample preparation, Writing—original draft, analysis. Deepak Kumar: Formal analysis and investigation, conceptualization and editing. Maitri Patel: Experimentation, analysis. D.K. Kanchan: Final draft, characterization. Kuldeep Mishra: Conceptualization, methodology. Mahendra Singh Rathore: Supervision, characterization.
Corresponding authors
Ethics declarations
Ethical approval
The authors approve that the manuscript follows the ethical guideline.
Consent for publication
The authors agree with the publishing policy and submit this manuscript in accordance with this policy.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Madhani, V., Kumar, D., Patel, M. et al. Studies on potassium-ion conducting gel polymer electrolytes containing poly(vinylidene fluoride-hexafluoropropylene) polymer, potassium permanganate salt added with different plasticizers. Ionics 30, 2139–2153 (2024). https://doi.org/10.1007/s11581-024-05405-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05405-8