Abstract
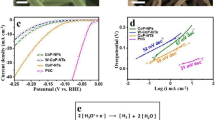
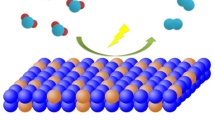
The development of inexpensive, highly effective and durable electrocatalysts for hydrogen production via water electrolysis is of paramount importance. Transition metal oxides, owing to their simplicity in preparation and cost-effectiveness, have been widely investigated, particularly cobalt, nickel, and manganese oxides. Inspired by the synergies of the ternary cathode materials of lithium-ion batteries (LiNixMnyCo1-x-yO2, NMC), a simple and feasible method was used to prepare a graphite plate as the support, and Ni and Mn were co-doped with Co3O4 as an efficient water decomposition catalyst. Experimental studies indicated that the 10% Ni and 10% Mn co-doped Co3O4 (Co2.4Ni0.3Mn0.3O4, CNM-811) exhibited the lowest overpotential in the oxygen evolution reaction (OER), with CNM-811 achieving an overpotential of 338 mV at 30 mA cm−2 in NaOH aqueous solution, the Tafel slope is 79.71 mV dec−1. In the hydrogen evolution reaction (HER), an overpotential of 190 mV was attained at 10 mA cm−2. In overall water splitting, a cell voltage of 2.048 V was achieved at 100 mA cm−2, which was superior to many analogous catalysts reported today.







Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Farias CBB, Barreiros RCS, Silva MFD, Casazza AA, Converti A, Sarubbo LA (2022) Use of hydrogen as fuel: a trend of the 21st century. Energies 15:311
Liu C, Ma H, Yuan M, Yu Z, Li J, Shi K, Liang Z, Yang Y, Zhu T, Sun G, Li H, Ma S (2018) (NiFe)S2 nanoparticles grown on graphene as an efficient electrocatalyst for oxygen evolution reaction. Electrochim Acta 286:195–204
Ye B, Huang L, Hou Y, Jiang R, Sun L, Yu Z, Zhang B, Huang Y, Zhang Y (2019) Pt (111) quantum dot decorated flower-like αFe2O3 (104) thin film nanosheets as a highly efficient bifunctional electrocatalyst for overall water splitting. J Mater Chem A 7:11379–11386
Vazhayil A, Ashok CS, Thomas N (2023) Probing the electrocatalytic activity of hierarchically mesoporous M-Co3O4 (M = Ni, Zn, and Mn) with branched pattern for oxygen evolution reaction. J Electroanal Chem 934:117298
Chi J, Yu H (2018) Water electrolysis based on renewable energy for hydrogen production. Chinese J Catal 39:390–394
Jiao S, Fu X, Wang S, Zhao Y (2021) Perfecting electrocatalysts via imperfections: towards the large-scale deployment of water electrolysis technology. Energy Environ Sci 14:1722–1770
Pham HQ, Huynh TT, Huynh Q (2023) Mixed-oxide-containing composite-supported MoPt with ultralow Pt content for accelerating hydrogen evolution performance. Chem Commun 59:215–218
Yin J, Jin J, Lin H, Yin Z, Li J, Lu M, Guo L, Xi P, Tang Y, Yan C-H (2020) Optimized metal chalcogenides for boosting water splitting. Adv Sci 7:1903070
Anantharaj S, Aravindan V (2020) Developments and perspectives in 3d transition-metal-based electrocatalysts for neutral and near-neutral water electrolysis. Adv Energy Mater 10:1902666
Zhang Z, Chen K, Zhao Q, Huang M, Ouyang X (2021) Electrocatalytic and photocatalytic performance of noble metal doped monolayer MoS2 in the hydrogen evolution reaction: A first principles study. Nano Mater Sci 3:89–94
Wang H, Yang P, Sun X, Xiao W, Wang X, Tian M, Xu G, Li Z, Zhang Y, Liu F, Wang L, Wu Z (2023) Co-Ru alloy nanoparticles decorated onto two-dimensional nitrogen doped carbon nanosheets towards hydrogen/oxygen evolution reaction and oxygen reduction reaction. J Energy Chem 87:286–294
Li Q, Luan X, Xiao Z, Xiao W, Xu G, Li Z, Wu Z, Wang L (2023) Ultrafast microwave synthesis of Ru-Doped MoP with abundant P vacancies as the electrocatalyst for hydrogen generation in a wide pH range. Inorg Chem 62:9687–9694
Wu Z, Yang P, Li Q, Xiao W, Li Z, Xu G, Liu F, Jia B, Ma T, Feng S, Wang L (2023) Microwave synthesis of Pt clusters on black TiO2 with abundant oxygen vacancies for efficient acidic electrocatalytic hydrogen evolution. Angew Chem Int Ed 62:e202300406
Liu G, Xu Y, Yang T, Jiang L (2023) Recent advances in electrocatalysts for seawater splitting. Nano Mater Sci 5:101–116
Chen Z, Li Q, Xiang H, Wang Y, Yang P, Dai C, Zhang H, Xiao W, Wu Z, Wang L (2023) Hierarchical porous NiFe-P@NC as an efficient electrocatalyst for alkaline hydrogen production and seawater electrolysis at high current density. Inorg Chem Front 10:1493–1500
Song M, Zhang Z, Li Q, Jin W, Wu Z, Fu G, Liu X (2019) Ni-foam supported Co(OH)F and Co-P nanoarrays for energy-efficient hydrogen production via urea electrolysis. J Mater Chem A 7:3697–3703
Song Y, Xie W, Shao M, Duan X (2023) Integrated electrocatalysts derived from metal organic frameworks for gas-involved reactions. Nano Mater Sci 5:161–176
Wu Z, Nie D, Song M, Jiao T, Fu G, Liu X (2019) Facile synthesis of Co-Fe-B-P nanochains as an efficient bifunctional electrocatalyst for overall water-splitting. Nanoscale 11:7506–7512
Zhong Y, Wang S, Zhang L (2023) In situ electrochemical metal (Co, Ni) oxide deposition on MoS2 nanosheets for highly efficient electrocatalytic water splitting. New J Chem 47:4430–4438
Li X, Liu Y, Sun Q, Huang W-H, Wang Z, Chueh C-C, Chen C-L, Zhu Z (2021) Surface engineered CoP/Co3O4 heterojunction for high-performance bi-functional water splitting electro-catalysis. Nanoscale 13:20281–20288
Tang D, Ma Y, Liu Y, Wang K, Liu Z, Li W, Li J (2022) Amorphous three-dimensional porous Co3O4 nanowire network toward superior OER catalysis by lithium-induced. J Alloys Compd 893:162287
Bhatti AL, Tahira A, Gradone A, Mazzaro R, Morandi V, Aftab U, Abro MI, Nafady A, Qi K, Infantes-Molina A, Vomiero A, Ibupoto ZH (2021) Nanostructured Co3O4 electrocatalyst for OER: The role of organic polyelectrolytes as soft templates. Electrochim Acta 398:139338
Min K, Hwang M, Shim SE, Lim D, Baeck S-H (2021) Defect-rich Fe-doped Co3O4 derived from bimetallic-organic framework as an enhanced electrocatalyst for oxygen evolution reaction. Chem Eng J 424:130400
Li G, Chen M, Ouyang Y, Yao D, Lu L, Wang L, Xia X, Lei W, Chen S-M, Mandler D, Hao Q (2019) Manganese doped Co3O4 mesoporous nanoneedle array for long cycle-stable supercapacitors. Appl Surf Sci 469:941–950
Xiong S, Weng S, Tang Y, Qian L, Xu Y, Li X, Lin H, Xu Y, Jiao Y, Chen J (2021) Mo-doped Co3O4 ultrathin nanosheet arrays anchored on nickel foam as a bi-functional electrode for supercapacitor and overall water splitting. J Colloid Interf Sci 602:355–366
Li S, Hao X, Abudula A, Guan G (2019) Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage: current status and perspectives. J Mater Chem A 7:18674–18707
Li L, Xu Q, Zhang Y, Li J, Fang J, Dai Y, Cheng X, You Y, Li X (2020) Low Ni-doped Co3O4 porous nanoplates for enhanced hydrogen and oxygen evolution reaction. J Alloys Compd 823:153750
Shen Y, Xue H, Wang S, Wang Z, Zhang D, Yin D, Wang L, Cheng Y (2021) A highly promising high-nickel low-cobalt lithium layered oxide cathode material for high-performance lithium-ion batteries. J Colloid Interf Sci 597:334–344
Venkatkarthick R, Niu J, Srikhaow A, Sriprachuabwong C, Vasudevan S, Tuantranont A, Qin J (2021) New insight into the electrocatalysis of Ni-Rich trimetallic NCM-based hydroxides for water oxidation. ACS Appl Energy Mater 4:6520–6530
Miao R, He J, Sahoo S, Luo Z, Zhong W, Chen S-Y, Guild C, Jafari T, Dutta B, Cetegen SA, Wang M, Alpay SP, Suib SL (2017) Reduced graphene oxide supported nickel-manganese-cobalt spinel ternary oxide nanocomposites and their chemical-converted sulfide nanocomposites as efficient electrocatalysts for alkaline water splitting. ACS Catal 7:819–832
Rebekah A, Ashok Kumar E, Viswanathan C, Ponpandian N (2020) Effect of cation substitution in MnCo2O4 spinel anchored over rGO for enhancing the electrocatalytic activity towards oxygen evolution reaction (OER). Int J Hydrogen Energ 45:6391–6403
Zeng T, Guo B, Xu Z, Mo F, Chen X, Wang L, Ding Y, Bai J (2023) Manageable bubble release through 3D printed microcapillary for highly efficient overall water splitting. Adv Sci 10:2207495
Lin J, Ding Y, Jin H, Zeng T (2023) Optimized Ni Co, Mn oxides anchored on graphite plates for highly efficient overall water splitting. Catalysts 13:1031
Ingier-Stocka E, Grabowska A (1998) Thermal analysis of cobalt(II) salts with some carboxylic acids. J Therm Anal Calorim 54:115–123
Wanjun T, Donghua C (2007) Mechanism of thermal decomposition of cobalt acetate tetrahydrate. Chem Pap 61:329–332
Gadalla AM, Yu H-F (1990) Thermal behavior of some NiII and FeIII salts. Thermochim Acta 164:21–36
Mohamed MA, Halawy SA, Ebrahim MM (1993) Non-isothermal decomposition of nickel acetate tetrahydrate. J Anal Appl Pyrol 27:109–110
Kremenović A, Jančar B, Ristić M, Vučinić-Vasić M, Rogan J, Pačevski A, Antić B (2012) Exchange-bias and grain-surface relaxations in nanostructured NiO/Ni induced by a particle size reduction. J Phys Chem C 116:4356–4364
Alnarabiji MS, Tantawi O, Ramli A, MohdZabidi NA, Ghanem OB, Abdullah B (2019) Comprehensive review of structured binary Ni-NiO catalyst: synthesis, characterization and applications. Renew Sus Energ Rev 114:109326
Mohamed MA, Halawy SA (1994) Kinetic and mechanistic study of the non-isothermal decomposition of manganese(II) acetate tetrahydrate. Thermochim Acta 242:173–186
Nohman AKH, Ismail HM, Hussein GAM (1995) Thermal and chemical events in the decomposition course of manganese compounds. J Anal Appl Pyrol 34:265–278
Chen J, Li L, Chen G, Peng J, Srinivasakannan C (2017) Rapid thermal decomposition of manganese ore using microwave heating. J Alloy Compd 699:430–435
Zhang R, Huang L, Yu Z, Jiang R, Hou Y, Sun L, Zhang B, Huang Y, Ye B, Zhang Y (2019) Spherical cactus-like composite based on transition metals Ni, Co and Mn with 1D / 2D bonding heterostructure for electrocatalytic overall water splitting. Electrochim Acta 323:134845
Zhu Z, Zhang H, Guo W, Zhou H, Zhou Y, Liang W, He M, Wei W, Yu T, Zhao H, Yang T (2023) Mo, V and M (M=Mn, Fe Co, Cu) Co-modulated Ni oxides in-situ derived from nickel foam as efficient electrocatalysts for alkaline hydrogen evolution and oxygen evolution. Mol Catal 54:113132
Tian R, Wang F, Zou C, Pei Z, Guo X, Yang H (2023) Modulating organic ligands to construct 2D–3D-hybrid porous P-doped metal-organic frameworks electrocatalyst for overall water splitting. J Alloys Compd 933:167670
BaratiDarband G, Aliofkhazraei M, Rouhaghdam AS (2019) Facile electrodeposition of ternary Ni-Fe-Co alloy nanostructure as a binder free, cost-effective and durable electrocatalyst for high-performance overall water splitting. J Colloid Interf Sci 547:407–420
Choi Y, Kim D, Lin L, Yan B, Hong H, Qin X, Piao Y (2021) CuFeN/CNT composite derived from kinetically modulated urchin-shaped MOF for highly efficient OER catalysis. Electrochim Acta 389:138637
Pu Z, Liu T, Zhang G, Chen Z, Li D-S, Chen N, Chen W, Chen Z, Sun S (2022) General synthesis of transition-metal-based carbon-group intermetallic catalysts for efficient electrocatalytic hydrogen evolution in wide pH range. Adv Energy Mater 12:2200293
Chen D, Yu R, Lu R, Pu Z, Wang P, Zhu J, Ji P, Wu D, Wu J, Zhao Y, Kou Z, Yu J, Mu S (2022) Tunable Ru-Ru2P heterostructures with charge redistribution for efficient pH-universal hydrogen evolution. InfoMat 4:e12287
Chen D, Yu R, Wu D, Zhao H, Wang P, Zhu J, Ji P, Pu Z, Chen L, Yu J, Mu S (2022) Anion-modulated molybdenum oxide enclosed ruthenium nano-capsules with almost the same water splitting capability in acidic and alkaline media. Nano Energy 100:107445
Zhao M, Zhou G, Liu X, Shen X, Lv J, Hu C, Wang Y, Tan W, Sun S, Ma Y, Wang C, Yang J, Zhang M, He G, Yang L (2021) One step hydrothermal synthesis of Ni-MoS2-RGO bifunctional electrocatalysts for HER and OER. Int J Electrochem Sci 16:210323
Fang W, Wang J, Hu Y, Cui X, Zhu R, Zhang Y, Yue C, Dang J, Cui W, Zhao H, Li Z (2021) Metal-organic framework derived Fe-Co-CN/reduced graphene oxide for efficient HER and OER. Electrochim Acta 365:137384
Zhu S, Lei J, Wu S, Liu L, Chen T, Yuan Y, Ding C (2022) Construction of Fe-Co-Ni-Sx/NF nanomaterial as bifunctional electrocatalysts for water splitting. Mater Lett 311:131549
Wang Q, Zhao S, Yu H, Zhang D, Wang Q (2022) Synergistic Engineering of Defects and Architecture in a Co@Co3O4@N-CNT Nanocage toward Li-Ion Batteries and HER. Inorg Chem 61:19567–19576
Peighambardoust NS, Sadeghi E, Mete B, BarisYagci M, Aydemir U (2022) Evaluating electrocatalytic activity of metal-substituted hafnium diboride (Hf1-xTMxB2; TM = Ni and Co) toward water splitting. J Alloys Compd 905:164148
Hasan MM, Gomaa AK, Khedr GE, Salem KE, Shaheen BS, Allam NK (2022) Highly durable compositionally variant bifunctional tetrametallic Ni–Co–Mn–Fe phosphide electrocatalysts synthesized by a facile electrodeposition method for high-performance overall water splitting. Energy Fuels 36:14371–14381
Wei D, Chen L, Tian L, Ramakrishna S, Ji D (2023) Melamine-derived carbon foam-supported graphene–CoNi nanocomposites as high-performance OER/HER bifunctional electrocatalysts. J Chem Technol Biotechnol 98:910–918
Wang Q, Xue Y, Sun S, Yan S, Miao H, Liu Z (2019) Facile synthesis of ternary spinel Co–Mn–Ni nanorods as efficient bi-functional oxygen catalysts for rechargeable zinc-air batteries. J Power Sources 435:226761
Shan X, Guo F, Page K, Neuefeind JC, Ravel B, Abeykoon AMM, Kwon G, Olds D, Su D, Teng X (2019) Framework doping of Ni enhances pseudocapacitive Na-Ion storage of (Ni)MnO2 layered birnessite. Chem Mater 31:8774–8786
Hsiao M-C, Liao S-H, Yen M-Y, Liu P-I, Pu N-W, Wang C-A, Ma C-CM (2010) Preparation of covalently functionalized graphene using residual oxygen-containing functional groups. ACS Appl Mater Interfaces 2:3092–3099
Priamushko T, Guggenberger P, Mautner A, Lee J, Ryoo R, Kleitz F (2022) Enhancing OER activity of Ni/Co oxides via Fe/Mn substitution within tailored mesoporous frameworks. ACS Appl Energy Mater 5:13385–13397
Acknowledgements
This work was supported by the Post Doctoral Funding Project of Zhejiang Province (No. ZJ2022023), National Natural Science Foundation of China (No. 22073069 and 21773082).
Author information
Authors and Affiliations
Contributions
Jie Lin: Methodology, Investigation, Formal Analysis, Data curation, Writing—original draft, Writing—review & editing. Yihong Ding: Conceptualization, Funding acquisition, Writing—review and editing. Huile Jin: Guidance on the use of some experimental instruments. Tianbiao Zeng: Methodology, Supervision, Project administration, Analysis, Writing—review.
Corresponding authors
Ethics declarations
Ethical approval
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, J., Ding, Y., Jin, H. et al. Trinary metal oxides of Co, Ni, and Mn as efficient overall water splitting catalyst. Ionics 30, 2287–2298 (2024). https://doi.org/10.1007/s11581-024-05396-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05396-6