Abstract
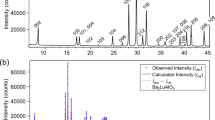
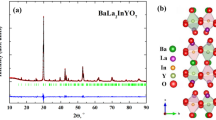
The perovskite-related oxides with intergrowth structure are a novel and promising class of protonic conductors. The development of this class of materials makes it possible to develop proton-conducting ceramics for intermediate temperatures (300–600 °C) for SOFCs (solid oxide fuel cells) applications. In this work, new complex oxide Ba5Y0.5In1.5Al2ZrO13 was obtained and investigated as a protonic conductor. Ba5Y0.5In1.5Al2ZrO13 shows the ability to water uptake and exhibits higher values of hydration degree (~ 0.40 mol H2O) than parent compound Ba5In2Al2ZrO13 (~ 0.30 mol H2O). IR spectroscopy confirmed the presence of OH−-groups in the hydrated phase Ba5Y0.5In1.5Al2ZrO13. The hydration ability is explained by the possibility of increasing the coordination number of barium in oxygen-deficient layers and the presence of sufficient space for the participation of OH−-groups in its coordination. Investigation of transport properties shows that in wet air (pH2O = 1.92·10−2 atm) below ~ 700 °C the conductivity is predominantly protonic. Proton mobility calculations show that the introduction of yttrium into the indium sublattice leads to an increase in mobility, which is probably due to an increase in the unit cell volume.











Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the authors upon reasonable request.
References
Steele BHC, Heinzel A (2010) Materials for fuel-cell technologies. Nat 414:345–352. https://doi.org/10.1142/9789814317665_0031
Norby T (2017) Advances in proton ceramic fuel cells, steam electrolyzers, and dehydrogenation reactors based on materials and process optimizations. ECS Trans 80:23–32. https://doi.org/10.1149/08009.0023ecst
Fabbri E, Pergolesi D, Traversa E (2010) Materials challenges toward proton-conducting oxide fuel cells: a critical review. Chem Soc Rev 39:4355–4369. https://doi.org/10.1039/b902343g
Zhang W, Hu Y (2021) Progress in proton-conducting oxides as electrolytes for low-temperature solid oxide fuel cells: From materials to devices. Energy Sci Eng 9:984–1011. https://doi.org/10.1002/ese3.886
Tarancón A (2009) Strategies for lowering solid oxide fuel cells operating temperature. Energies 2:1130–1150. https://doi.org/10.3390/en20401130
Meng Y, Gao J, Zhao Z, Amoroso J, Tong J, Brinkman KS (2019) Review: recent progress in low-temperature proton-conducting ceramics. J Mater Sci 54:9291–9312. https://doi.org/10.1007/s10853-019-03559-9
Wachsman ED, Lee KT (2010) Lowering the temperature of solid oxide fuel cells. Sci 334:935–939. https://doi.org/10.1126/science.1204090
Medvedev D (2019) Trends in research and development of protonic ceramic electrolysis cells. Int J Hydrog Energy 44:26711–26740. https://doi.org/10.1016/j.ijhydene.2019.08.130
Jing Y, Matsumoto H, Aluru N (2018) Mechanistic insights into hydration of solid oxides. Chem Mater 30:138–144. https://doi.org/10.1021/acs.chemmater.7b03476
Putilov L, Tsidilkovski V (2019) Impact of bound ionic defects on hydration of acceptor-doped proton-conducting perovskites. Phys Chem Chem Phys 21:6391–6406. https://doi.org/10.1039/C8CP07745B
Kim J, Sengodan S, Kim S, Kwon O, Bud Y, Kim G (2019) Proton conducting oxides: a review of materials and applications for renewable energy conversion and storage. Renew Sustain Energy Rev 109:606–618. https://doi.org/10.1016/j.rser.2019.04.042
Kuzmin AV, Stroeva AYu, Gorelov VP, Novikova YuV, Lesnichyova AS, Farlenkov AS, Khodimchuk AV (2019) Synthesis and characterization of dense protonconducting La1-xSrxScO3-α ceramics. Int J Hydrog Energy 44:1130–1138. https://doi.org/10.1016/j.ijhydene.2018.11.041
Kuzmin AV, Lesnichyova AS, Tropin ES, Stroeva AYu, Vorotnikov VA, Solodyankina DM, Belyakov SA, Plekhanov MS, Farlenkov AS, Osinkin DA, Beresnev SM, Ananyev MV (2020) LaScO3-based electrolyte for protonic ceramic fuel cells: Influence of sintering additives on the transport properties and electrochemical performance. J Power Sources 466:228255. https://doi.org/10.1016/j.jpowsour.2020.228255
Hossain MK, Biswas MC, Chanda RK, Rubel MHK, Khan MI, Hashizume K (2021) A review on experimental and theoretical studies of perovskite barium zirconate proton conductors. Emergent Mater 4:999–1027. https://doi.org/10.1007/s42247-021-00230-5
Fronzi M, Tateyama Y, Marzari N, Nolan M, Traversa E (2016) First-principles molecular dynamics simulations of proton diffusion in cubic BaZrO3 perovskite under strain conditions. Mater Renew Sustain Energy 5:14. https://doi.org/10.1007/s40243-016-0078-9
Kochetova N, Animitsa I, Medvedev D, Demin A, Tsiakaras P (2016) Recent activity in the development of proton-conducting oxides for high-temperature applications. RSC Adv 6:73222–73268. https://doi.org/10.1039/C6RA13347A
Fisher C, Islam M (1999) Defect, protons and conductivity in brownmillerite-structured Ba2In2O5. Solid State Ionics 118:355–363. https://doi.org/10.1016/S0167-2738(98)00391-9
Jalarvo N, Haavik C, Kongshaug C, Norby P, Norby T (2009) Conductivity and water uptake of Sr4(Sr2Nb2)O11·nH2O and Sr4(Sr2Ta2)O11·nH2O. Solid State Ionics 180:1151–1156. https://doi.org/10.1016/j.ssi.2009.05.021
Tarasova N, Animitsa, (2022) I Materials AIILnInO4 with Ruddlesden-Popper structure for electrochemical applications: relationship between ion (oxygen-ion, proton) conductivity, water uptake, and structural changes. Materials 15:114. https://doi.org/10.3390/ma15010114
Zhou Y, Shiraiwa M, Nagao M, Fujii K, Tanaka I, Yashima M, Baque L, Basbus J, Mogni L, Skinner S (2021) Protonic conduction in the BaNdInO4 structure achieved by acceptor doping. Chem Mater 33:2139–2146. https://doi.org/10.1021/acs.chemmater.0c04828
Gurudeo N, Dharmendra Y, Shail U (2020) Ruddlesden-Popper phase A2BO4 oxides: recent studies on structure, electrical, dielectric, and optical properties. J Adv Ceram 9:29–148. https://doi.org/10.1007/s40145-020-0365-x
Armstrong AR, Anderson PA (1994) Synthesis and structure of a new layered niobium blue bronze: Rb2LaNb2O7. Inorg Chem 33:4366–4369. https://doi.org/10.1021/ic00097a026
Hayden AE, Lingling M, Ram S, Anthony KC (2021) Layered Double Perovskites. Annu Rev Mater Res 51:351–380. https://doi.org/10.1146/annurev-matsci-092320-102133
Fop S, McCombie K, Wildman E, Skakle J, Irvine J, Connor P, Savaniu C, Ritter C, Mclaughlin A (2020) High oxide ion and proton conductivity in a disordered hexagonal perovskite. Nat Mater 19:752–757. https://doi.org/10.1038/s41563-020-0629-4
Fop S, Dawson J, Fortes A, Ritter C, Mclaughlin A (2021) Hydration and ionic conduction mechanisms of hexagonal perovskite derivatives. Chem Mater 33:4651–4660. https://doi.org/10.1021/acs.chemmater.1c01141
Unti LFK, Grzebielucka EC, Chinelatto ASA, Mather GC, Chinelatto AL (2019) Synthesis and electrical characterization of Ba5Nb4O15 and Ba5Nb3.9M0.1O(15-δ) (M= Ti, Zr) hexagonal perovskites. Ceram Int 45:5087–5092. https://doi.org/10.1016/j.ceramint.2018.11.211
Tabacaru C, Aguadero A, Sanz J, Chinelatto AL, Thursfield A, Pérez-Coll D, Mather GC (2013) Protonic and electronic defects in the 12R-type hexagonal perovskite Sr3LaNb3O12. Solid State Ionics 253:239–246. https://doi.org/10.1016/j.ssi.2013.10.031
Murakami T, Hester J, Yashima M (2020) high proton conductivity in Ba5Er2Al2ZrO13, a hexagonal perovskite-related oxide with intrinsically oxygen-deficient layers. J Am Chem Soc 142:11653–11657. https://doi.org/10.1021/jacs.0c02403
Andreev R, Korona D, Anokhina I, Animitsa I (2022) Proton and oxygen-ion conductivities of hexagonal perovskite Ba5In2Al2ZrO13. Materials 15:3944. https://doi.org/10.3390/ma15113944
Andreev RD, Korona DV, Anokhina IA, Animitsa IE (2022) Novel Nb5+-doped hexagonal perovskite Ba5In2Al2ZrO13 (structure, hydration, electrical conductivity). Chimica Techno Acta 9:20229414. https://doi.org/10.15826/chimtech.2022.9.4.14
Shpanchenko R, Abakumov A, Antipov E, Kovba L (1994) Crystal structure of Ba5In2Al2ZrO13. J Alloy Compd 206:185–188. https://doi.org/10.1016/0925-8388(94)90033-7
Shpanchenko RV, Abakumov AM, Antipov EV, Nistor L, Van Tendeloo G, Amelinckx S (1995) Structural study of the new complex oxides Ba5-ySryR2-xAl2Zr1+xO13+x/2 (R = Gd-Lu, Y, Sc). J Solid State Chem 118:180–192. https://doi.org/10.1006/jssc.1995.1329
Fop S, McCombie KS, Wildman EJ, Skakle JMS, Mclaughlin AC (2019) Hexagonal perovskite derivatives: a new direction in the design of oxide ion conducting materials. Chem Commun 55:2127–2137. https://doi.org/10.1039/C8CC09534E
Shannon R (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A Cryst Phys Diffr Theor Gen Crystallogr 32:751–767. https://doi.org/10.1107/S0567739476001551
Nakamoto K (1986) Infrared and Raman spectra of inorganic and coordination compounds. Wiley, New York
Yukhnevich GV (1973) Infrared spectroscopy of water. Nauka, Moscow (in Russian)
Colomban P, Romain F, Neiman A, Animitsa I (2001) Double perovskites with oxygen structural vacancies: Raman spectra, conductivity and water uptake. Solid State Ionics 145:339–347. https://doi.org/10.1016/S0167-2738(01)00929-8
Bhella SS, Thangadurai V (2009) Synthesis and characterization of carbon dioxide and boiling water stable proton conducting double perovskite-type metal oxides. J Power Sources 186:311–319. https://doi.org/10.1016/j.jpowsour.2008.09.110
Tarasova NA, Galisheva AO, Animitsa IE (2019) The local structure and hydration processes of halogen-substituted perovskites based on Ba4In2Zr2O11. Opt Spectrosc 126:336–340. https://doi.org/10.1134/S0030400X19040222
Novak A (1974) Hydrogen bonding in solids correlation of spectroscopic and crystallographic data. In: Large molecules. structure and bonding, vol 18. Springer Berlin, Heidelberg, pp 177–217 https://doi.org/10.1007/BFb0116438
Kreuer KD (1999) Aspects of the formation and mobility of protonic charge carriers and the stability of perovskite-type oxides. Solid State Ionics 125:285–302. https://doi.org/10.1016/S0167-2738(99)00188-5
Haugsrud R (2016) High temperature proton conductors – fundamentals and functionalities. In: Diffusion foundations, vol 8. Trans Tech Publications Ltd, Stäfa, pp 31–79 https://doi.org/10.4028/www.scientific.net/DF.8.31
Kreuer KD, Fuchs A, Maier J (1992) HD isotope effect of proton conductivity and proton conduction mechanism in oxides. Solid State Ionics 77:157–162. https://doi.org/10.1016/0167-2738(94)00265-T
Kreuer K, Adams S, Fuchs W, Klock U, Maier J (2001) Proton conducting alkaline earth zirconates and titanates for high drain electrochemical applications. Solid State Ionics 145:295–306. https://doi.org/10.1016/S0167-2738(01)00953-5
Matsumoto H, Kawasaki Y, Ito N, Enoki M, Ishihara T (2007) Relation between electrical conductivity and chemical stability of BaCeO3-based proton conductors with different trivalent dopants, electrochem. Solid-State Lett 10:B77–B80. https://doi.org/10.1149/1.2458743
Tarasova N, Bedarkova A (2022) Advanced Proton-Conducting Ceramics Based on Layered Perovskite BaLaInO4 for Energy Conversion Technologies and Devices. Materials 15:6841. https://doi.org/10.3390/ma15196841
Okuyama Y, Kozai T, Ikeda S, Matsuka M, Takaaki S, Matsumoto H (2014) Incorporation and conduction of proton in Sr-doped LaMO3 (M=Al, Sc, In, Yb, Y). Electrochim Acta 125:443–449. https://doi.org/10.1016/j.electacta.2014.01.113
Funding
This work was supported by the Russian Science Foundation and the Government of Sverdlovsk region, Joint Grant 22–23-20003 https://rscf.ru/en/project/22-23-20003/
Author information
Authors and Affiliations
Contributions
Andreev Roman: methodology, investigation, formal analysis, writing—original draft preparation, writing—review and editing. Animitsa Irina: conceptualization, methodology, writing—original draft preparation, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andreev, R.D., Animitsa, I.E. Protonic transport in the novel complex oxide Ba5Y0.5In1.5Al2ZrO13 with intergrowth structure. Ionics 29, 4647–4658 (2023). https://doi.org/10.1007/s11581-023-05187-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05187-5