Abstract
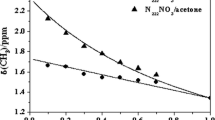
This study explores the vibrational and nuclear magnetic resonance spectra of free ions as well as those coordinated with sodium ions, which are commonly utilized to study ion-ion and ion-solvent interactions in electrolytic solutions of dipolar aprotic solvents. The spectral investigations suggested the origin of new bands due to solvent shell of sodium ions. The ion-solvent interactions were confirmed by the carbonyl symmetric stretch ring deformation bands in N,N-dimethylacetamide. The probability of new contact ion pairs between sodium and perchlorate ions with N,N-dimethylacetamide (DMA) increases on enhancing the increase in electrolytic concentration of sodium perchlorate in binary solvent mixtures. FTIR vibrational spectra and 13C-NMR spectra of several electrolytic concentration solutions of sodium perchlorate (NaClO4) were studied in pure DMA and its binary mixture with 2-aminoethanol (AE). The study investigates the vibrational spectra of free ions as well as those coordinated with Na+ ions, which are widely used to study ion-ion and ion-solvent interactions in electrolytic solutions of dipolar aprotic solvents. The solutions of the salt at different concentrations were prepared in the molar ratio of NaClO4/AE/DMA (4:4:4) from 0.25 to 1.0 m. The vibrational spectroscopic and 13C-NMR studies suggested the origin of new bands due to solvent shell of Na+ ions, which is due to O=C–N deformation behavior. The study revealed that the Na+ ions interacted through the coordinate bond to both N-atom of NH4+ structure as well as oxygen atom of the C=O (carbonyl group) of amide. The interaction between Na+ ions and solvent suggests preferential solvated by DMA in the binary solvent mixtures. The ion-solvent interactions were confirmed by the carbonyl symmetric stretch ring deformation bands in DMA. The new contact ion-pairs between Na+ and ClO4− ions are more probable with increase in the concentration of sodium perchlorate in binary solvent mixtures.
Graphical abstract







Similar content being viewed by others
References
Vedenyapina MD, Kulova TL, Kudryashova YO et al (2020) Interaction between sodium and dimethylacetamide as the cause of instability in the operation of a sodium-ion battery. Russian J Phys Chem A 94:1276–1279
Lewis IR, Edwards H (2001) Handbook of Raman spectroscopy: from the research laboratory to the process line. CRC press
Müller HSP, Halfen DT, Ziurys LM (2012) The spectroscopic parameters of sodium cyanide, NaCN (X 1A′), revisited. J Mol Spect 272:23–26
Xuan X, Zhang H, Wang J, Wang H (2003) Raman spectroscopic and DFT studies on solutions of NaBF 4 in N,N-dimethylformamide. J Raman Spectrosc 34:465–470. https://doi.org/10.1002/jrs.1026
dos Santos JD, da Silva ALL, da Luz CJ et al (2013) Development of a vinasse nutritive solution for hydroponics. J Environ Manag 114:8–12
Fawcett WR, Liu G (1992) A study of ion pairing in acetonitrile solutions containing magnesium perchlorate using attenuated total reflection FTIR spectroscopy. J Phys Chem 96:4231–4236
Verbovy DM, Smagala TG, Brynda MA, Fawcett WR (2006) A FTIR study of ion-solvent interactions in N,N-dimethylacetamide. J Mol Liq 129:13–17. https://doi.org/10.1016/j.molliq.2006.08.008
Cha DK, Kloss AA, Tikanen AC, Fawcett WR (1999) Solvent-induced frequency shifts in the infrared spectrum of acetone in organic solvents. Phys Chem 1:4785–4790
Fawcett WR, Brooksby P, Verbovy D et al (2005) Studies of anion solvation in polar aprotic solvents. J Mol Liq 118:171–178
Fawcett WR, Liu G, Kessler TE (1993) Solvent-induced frequency shifts in the infrared spectrum of acetonitrile in organic solvents. J Phys Chem 97:9293–9298
Brooksby PA, Fawcett WR (2006) Infrared (attenuated total reflection) study of propylene carbonate solutions containing lithium and sodium perchlorate. Spectroc Acta Part A: Mol Biomol Spectrosc 64:372–382
Reichardt C, Welton T (2011) Solvents and solvent effects in organic chemistry. Wiley
Nazri M (2009) Liquid electrolytes: some theoretical and practical aspects. In: Lithium batteries. Springer, pp 509–529
Fawcett WR (2004) Liquids, solutions, and interfaces: from classical macroscopic descriptions to modern microscopic details. Oxford University Press
Chen S, Sun P, Humphreys J et al (2021) N, N-dimethylacetamide-diluted nitrate electrolyte for aqueous Zn//LiMn2O4 hybrid ion batteries. ACS Appl Mater Int 13:46634–46643
Lavanya R, Natchimuthu N (2021) Effect of N, N-dimethylacetamide/lithium chloride modified microcrystalline cellulose (MCC) on the processing behaviour and properties of celluloserubber (NBR and EPDM) composites. J Polym Mater 38:79–100
Vraneš M, Tot A, Zec N et al (2014) Volumetric properties of binary mixtures of 1-butyl-3-methylimidazolium tris (pentafluoroethyl) trifluorophosphate with N-methylformamide, N-ethylformamide, N, N-dimethylformamide, N, N-dibutylformamide, and N, N-dimethylacetamide from (293.15 to 323.15). J Chem Eng Data 59:3372–3379
Huo Y, Xia S, Ma P (2007) Densities of ionic liquids, 1-butyl-3-methylimidazolium hexafluorophosphate and 1-butyl-3-methylimidazolium tetrafluoroborate, with benzene, acetonitrile, and 1-propanol at T=(293.15 to 343.15) K. J Chem Eng Data 52:2077–2082
Gadžurić S, Tot A, Zec N et al (2014) Volumetric properties of binary mixtures of 1-butyl-1-methylpyrrolidinium tris (pentafluoroethyl) trifluorophosphate with N-methylformamide, N-ethylformamide, N, N-dimethylformamide, N, N-dibutylformamide, and N, N-dimethylacetamide from (293.15 to 323.15). J Chem Eng Data 59:1225–1231
Wu J-Y, Chen Y-P, Su C-S (2015) Density and viscosity of ionic liquid binary mixtures of 1-n-butyl-3-methylimidazolium tetrafluoroborate with acetonitrile, N, N-dimethylacetamide, methanol, and N-methyl-2-pyrrolidone. J Sol Chem 44:395–412
Da Silva EF, Alves WA (2012) Vibrational study on the solvates formed during interactions of amide with alkaline earth metal ions. Vibr Spectrosc 62:264–267
Phadagi R, Singh S, Hashemi H et al (2021) Understanding the role of dimethylformamide as co-solvents in the dissolution of cellulose in ionic liquids: experimental and theoretical approach. J Mol Liq 328:115392
Kumari R, Mishra RC, Sheoran R, Yadav JP (2020) Fractionation of antimicrobial compounds from Acacia nilotica twig extract against oral pathogens. Biointer Res Appl Chem 10:7097–7105. https://doi.org/10.33263/BRIAC106.70977105
Park J-R, Verderosa AD, Totsika M et al (2021) Thermoresponsive polymer–antibiotic conjugates based on gradient copolymers of 2-oxazoline and 2-oxazine. Biomacromolecules 22:5185–5194
Jawaid M, Ahmad A, Reddy AVB (2021) Ionic liquid-based technologies for environmental sustainability. Elsevier
Inada Y, Niwa Y, Iwata K et al (2006) Solvation structure of metal ions in nitrogen-donating solvents. J Mol Liq 129:18–24. https://doi.org/10.1016/j.molliq.2006.08.009
Alves WA, Téllez Soto CA, Hollauer E, Faria RB (2005) Vibrational studies on the selective solvation of Na+ and ClO3− ions in dimethylformamide–formamide mixture. Spectroc Acta Part A: Mol Biomol Spectrosc 62:755–760. https://doi.org/10.1016/J.SAA.2005.02.044
Gill DS, Anand H, Puri JK (2004) Study of the comparative solvation behaviour of Na+ and Cu+ cations in acetonitrile+ N, N-dimethylformamide mixtures at 298.15 K. Zeitschrift für Naturforschung A 59:615–620
Vineeth SK, Soni CB, Sun Y et al (2022) Implications of Na-ion solvation on Na anode–electrolyte interphase. Trends Chem 4:48–59
Li L, Chen X, Yuan Q et al (2022) Effects of minor mechanical deformation on the lifetime and performance of commercial 21700 lithium-ion battery. J Electrochem Soc 169:60544. https://doi.org/10.1149/1945-7111/ac79d4
Delmas C (2018) Sodium and sodium-ion batteries: 50 years of research. Adv Energy Mater 8:1703137
Chen W, Chen X, Qiao R et al (2022) Understanding the role of nitrogen and sulfur doping in promoting kinetics of oxygen reduction reaction and sodium ion battery performance of hollow spherical graphene. Carbon 187:230–240
Peters JF, Peña Cruz A, Weil M (2019) Exploring the economic potential of sodium-ion batteries. Batteries 5:10
Abraham KM (2020) How comparable are sodium-ion batteries to lithium-ion counterparts? ACS Energy Lett 5:3544–3547
Choudhary N, Li C, Moore J et al (2017) Asymmetric supercapacitor electrodes and devices. Adv Mater 29:1605336
Yao B, Zhang J, Kou T et al (2017) Paper-based electrodes for flexible energy storage devices. Adv Sci 4:1700107
Chen L, Li W, Wang Y et al (2014) Polyimide as anode electrode material for rechargeable sodium batteries. RSC Adv 4:25369–25373
Xu Y, Zhou M, Lei Y (2018) Organic materials for rechargeable sodium-ion batteries. Mater Today 21:60–78
Zhao Q, Lu Y, Chen J (2017) Advanced organic electrode materials for rechargeable sodium-ion batteries. Adv Energy Mater 7:1601792
Jones G, Dole M (1929) The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J Am Chem Soc 51:2950
Kaminsky M (1957) Ion-solvent interaction and the viscosity of strong-electrolyte solutions. Dis Faraday Soc 24:171–179
Falkenhagen H, Vernon EL (1932) LXII. The viscosity of strong electrolyte solutions according to electrostatic theory. London, Edinburgh, Dublin Philosop Magazine J Sci 14:537–565
Gill D, Society AS-J of the C, Faraday undefined, (1982) undefined acetone+ NN-dimethylformamide solvent system. Part 3.—Viscosity measurements of some electrolytes in acetone, NN-dimethylformamide and acetone+ NN. pubs.rsc.org
Gill D, Pathania V, Kumari A, et al Compressibility behaviour of some copper (I) perchlorate complexes in binary mixtures of acetonitrile with nitromethane and dimethylsulfoxide at 298.15 K. degruyter.com
Lind JE, Zwolenik JJ, Fuoss RM (1959) Calibration of conductance cells at 25° with aqueous solutions of potassium chloride. J Am Chem Soc 81:1557–1559. https://doi.org/10.1021/ja01516a010
Bockris JO, Reddy AKN (1998) Ion-solvent interactions. Modern. Electrochemistry 1:35
Robinson RA, Stokes RH (2002) Electrolyte solutions. Courier Corporation
Morales D, Chagas LG, Paterno D et al (2021) Transport studies of NaPF6 carbonate solvents-based sodium ion electrolytes. Electrochimica Acta 377:138062. https://doi.org/10.1016/j.electacta.2021.138062
Chagnes A, Carré B, Willmann P, Lemordant D (2001) Ion transport theory of nonaqueous electrolytes. LiClO4 in γ-butyrolactone: the quasi lattice approach. Electrochimica Acta 46:1783–1791
Kuratani K, Kishimoto I, Nishida Y et al (2016) Transport phenomena of nonaqueous electrolyte solutions at high concentrations: a comparison between the Li-and Na-systems. J Electrochem Soc 163:H417
Seo DM, Reininger S, Kutcher M et al (2015) Role of mixed solvation and ion pairing in the solution structure of lithium ion battery electrolytes. J Phys Chem C 119:14038–14046
Kumar S, Singh N, Anand H (2021) Ion-solvent interaction behaviour of lithium perchlorate in N,N-dimethylacetamide and its binary mixtures with 2-aminoethanol: FTIR vibrational and NMR spectroscopy study. Asian J Chem 33:1415–1419
Sajeevkumar VA, Singh S (1996) Infrared spectral studies on preferential solvation of lithium ions in binary mixtures of acetonitrile and dimethyl formamide. J Mol Struct 382:101–110
Xuan X, Wang J, Zhao Y, Zhang H (2005) FT-Raman spectroscopic evidences for the preferential solvation of sodium tetrafluorobrate in acetonitrile-based mixed solvents. Spectroc Acta Part A: Mol Biomol Spectrosc 62:500–505
Akopyan S, Bertagnolli H, Boyarskaya I et al (2001) IR spectra and microstructure of electrolyte solutions. Dependence of spectroscopic characteristics of solvated molecules on composition of solvates in the system CH 3 CN–Mg (ClO 4) 2–DMF. Phys Chem Chem Phys 3:2098–2104
Wang Z, Huang B, Lu Z et al (1996) Vibrational spectroscopic studies of interactions between LiClO4 and the plasticizer dimethylformamide. Solid State Ionics 92:265–271
Roeges NPG, Baas JMA (1994) A guide to the complete interpretation of infrared spectra of organic structures. Wiley, New York
Mielke Z, Barnes AJ (1986) Infrared matrix isolation studies of complexes between N, N-dimethylacetamide and hydrogen halides. Part 1.—Hydrogen chloride and hydrogen bromide complexes. J Chem Soc Faraday Trans 2: Mol Chem Phys 82:437–446
Jamróz D, Wójcik M, Lindgren J, Stangret J (1997) Solvation of Al3+, Fe3+, and Cr3+ cations in water− acetonitrile mixtures studied by IR spectroscopy. J Phys Chem B 101:6758–6762
Arivazhagan G, Parthipan G, Thenappan T (2009) Solute–solvent interactions of acid–1, 4-dioxane mixtures—by dielectric, FTIR, UV–vis and 13C NMR spectrometric methods. Spectroc Acta Part A: Mol Biomol Spectrosc 74:860–868
Wang J, Wu Y, Xuan X, Wang H (2002) Ion–molecule interactions in solutions of lithium perchlorate in propylene carbonate+ diethyl carbonate mixtures: an IR and molecular orbital study. Spectroc Acta Part A: Mol Biomol Spectrosc 58:2097–2104
RonaldáFawcett W (1993) Attenuated total reflection fourier-transform infrared spectroscopic study of ion–solvent and ion–ion interactions in alkali-metal perchlorate–acetonitrile solutions. J Chem Soc , Faraday Trans 89:811–816
James DW, Mayes RE (1984) Ion-ion-solvent interactions in solution. 8. Spectroscopic studies of the lithium perchlorate/N,N-dimethylformamide system. J Phys Chem 88:637–642. https://doi.org/10.1021/j150647a058
James DW, Mayes RE (1982) Ion-ion-solvent interactions in solution. I. Solutions of LiClO4 in acetone. Aust J Chem 35:1775–1784
Seo J-S, Cheong B-S, Cho H-G (2002) Solvation of LiClO4 and NaClO4 in deuterated acetonitrile studied by means of infrared and Raman spectroscopy. Spectroc Acta Part A: Mol Biomol Spectrosc 58:1747–1756
Denisova AS, Lysinova MB (2002) IR study of complexation in the system 1, 10-phenanthroline-LiClO4 (NaClO4)-acetone. Russian J Gen Chem 72:823–833
Salomon M (2009) ELECTROLYTES | Overview. In Garche JBT-E of EPS (ed). Elsevier. Amsterdam, 134–139
Perelygin IS, Klimchuk MA (1976) Infrared spectra of coordinated pyridine. J Appl Spectrosc 24:43–46
Mikhailov GP (2014) Calculation of vibrational spectra of coordinated perchlorate ion in dipolar aprotic solvents. J Appl Spectrosc 81:547–552
Perelygin IS, Klimchuk MA, Plakhotnik VN, Tovmash NF (1989) Infrared spectra and ion association in solutions of lithium tetrafluoroborate and perchlorate in 1.2-dimethoxyethane. Zhurnal Neorganicheskoj Khimii 34(9):2296–2298
Batkowska A, Szymański G, Werblan L (1990) Conductivities of lithium salts in a mixed solvent: comparison of conductance equations for their analysis. J Electroanal Chem Int Electrochem 287:229–238
Tsunekawa H, Narumi A, Sano M et al (2003) Solvation and ion association studies of LiBF4− propylenecarbonate and LiBF4− propylenecarbonate− trimethyl phosphate solutions. J Phys Chem B 107:10962–10966
Yang L, Xiao A, Lucht BL (2010) Investigation of solvation in lithium ion battery electrolytes by NMR spectroscopy. J Mol Liq 154:131–133
Reddy VP, Smart MC, Chin KB et al (2005) 13C NMR spectroscopic, CV, and conductivity studies of propylene carbonate-based electrolytes containing various lithium salts. Electrochem Solid State Lett 8:A294
Xin N, Sun Y, He M et al (2018) Solubilities of six lithium salts in five non-aqueous solvents and in a few of their binary mixtures. Fluid Phase Equil 461:1–7
Hayamizu K (2012) Temperature dependence of self-diffusion coefficients of ions and solvents in ethylene carbonate, propylene carbonate, and diethyl carbonate single solutions and ethylene carbonate+ diethyl carbonate binary solutions of LiPF6 studied by NMR. J Chem Eng Data 57:2012–2017
Peng J, Carbone L, Gobet M et al (2016) Natural abundance oxygen-17 NMR investigation of lithium ion solvation in glyme-based electrolytes. Electrochimica Acta 213:606–612
Acknowledgements
Mr. Suresh Kumar is thankful to the Department of Chemistry, Kurukshetra University, Kurukshetra (Haryana), for conducting the IR and NMR spectroscopy research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Anand, H. FTIR vibrational and 13C NMR spectroscopic study of the effect on ionic transport behavior of NaClO4 in N,N-dimethylacetamide and its binary mixtures with 2-aminoethanol. Ionics 29, 3143–3154 (2023). https://doi.org/10.1007/s11581-023-05069-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05069-w