Abstract
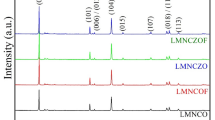
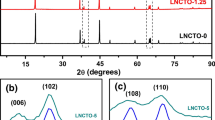
Ni-rich cathode materials LiNixCoyMn1-x-yO2 (NCM) are widely used in Li-ion batteries because of their high energy density and low material cost. However, the cycle and safety performance are poor due to internal structure instability, cation mixing, and surface instability. The Al-O bond and TM-F bond are higher than the TM-O bond, which is beneficial to the stable layered structure of NCM cathode material. In this paper, Ni-rich layered Li[(Ni0.9Co0.05Mn0.03)1-xAlx]O2-zFz (NCMAF) cathode material was prepared by a high-temperature solid-state method. When compared to NCM, the co-doped NCMAF shows excellent rate performance with the discharge capacity (177 mAh/g) at 10C and better cycle performance with a capacity retention of 72.7% after 200 cycles. According to XRD, Rietveld refined, and TEM analysis, the lithium layer distance of co-doped NCMAF is broader than that of pristine NCM. The capacity differential (dQ/dV) proves that the H2-H3 phase transition is inhibited during the charging and discharging process of NCMAF. Analysis of EIS reveals that the modified NCMAF has lower polarization and increases the diffusion coefficient (8.59 × 10−14 cm2/s) of lithium-ion than pristine NCM.










Similar content being viewed by others
Data availability
All data generated and analyzed during this study are included in this article.
References
Park G-T, Namkoong B, Kim S-B, Liu J, Yoon CS, Sun Y-K (2022) Introducing high-valence elements into cobalt-free layered cathodes for practical lithium-ion batteries. Nat Energy 7(10):946–954
Liu W, Oh P, Liu X, Lee MJ, Cho W, Chae S, Kim Y, Cho J (2015) Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem Int Ed 54(15):4440–4457
Ryu H-H, Sun HH, Myung S-T, Yoon CS, Sun Y-K (2021) Reducing cobalt from lithium-ion batteries for the electric vehicle era. Energy Environ Sci 14(2):844–852
Yan P, Zheng J, Chen T, Luo L, Jiang Y, Wang K, Sui M, Zhang J-G, Zhang S, Wang C (2018) Coupling of electrochemically triggered thermal and mechanical effects to aggravate failure in a layered cathode. Nat Commun 9(1):2437
Zhang R, Wang C, Zou P, Lin R, Ma L, Yin L, Li T, Xu W, Jia H, Li Q (2022) Compositionally complex doping for zero-strain zero-cobalt layered cathodes. Nature 610(7930):67–73
Hu G, Zhang Z, Li T, Gan Z, Du K, Peng Z, Xia J, Tao Y, Cao Y (2020) In situ surface modification for improving the electrochemical performance of Ni-rich cathode materials by using ZrP2O7. Chemsuschem 13(6):1603–1612
Shi J-L, Qi R, Zhang X-D, Wang P-F, Fu W-G, Yin Y-X, Xu J, Wan L-J, Guo Y-G (2017) High-thermal-and air-stability cathode material with concentration-gradient buffer for Li-ion batteries. ACS Appl Mater Interfaces 9(49):42829–42835
Hu G, Zhang S, Du K, Peng Z, Fang Z, Li L, Zhang Y, Huang J, Guan D, Huang M (2022) Enhanced cycle performance and synthesis of LiNi0.6Co0.2Mn0.2O2 single-crystal through the assist of Ba ion. J. Power Sources 542:231784
Liu W, Li X, Xiong D, Hao Y, Li J, Kou H, Yan B, Li D, Lu S, Koo A (2018) Significantly improving cycling performance of cathodes in lithium ion batteries: the effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2. Nano Energy 44:111–120
Liang J-Y, Zeng X-X, Zhang X-D, Wang P-F, Ma J-Y, Yin Y-X, Wu X-W, Guo Y-G, Wan L-J (2018) Mitigating interfacial potential drop of cathode-solid electrolyte via ionic conductor layer to enhance interface dynamics for solid batteries. J Am Chem Soc 140(22):6767–6770
Lee G, Choi Y, Ji H, Kim JY, Kim JP, Kang J, Kwon O, Kim DW, Park JH (2023) Interwoven carbon nanotube-poly (acrylic acid) network scaffolds for stable Si microparticle battery anode. Carbon 202:12–19
Cheng L, Zhang B, Su S-L, Ming L, Zhao Y, Tan X-X (2021) Al-doping enables high stability of single-crystalline LiNi0.7Co0.1Mn0.2O2 lithium-ion cathodes at high voltage. RSC advances 11(1):124–128
Lv Y, Cheng X, Qiang W, Huang B (2020) Improved electrochemical performances of Ni-rich LiNi0.83Co0.12Mn0.05O2 by Mg-doping. J. Power Sources 450:227718
Cheng Y, Sun Y, Chu C, Chang L, Wang Z, Zhang D, Liu W, Zhuang Z, Wang L (2022) Stabilizing effects of atomic Ti doping on high-voltage high-nickel layered oxide cathode for lithium-ion rechargeable batteries. Nano Res 15(5):4091–4099
Li Q, Li Z, Wu S, Wang Z, Liu X, Li W, Li N, Wang J, Zhuang W (2020) Utilizing diverse functions of zirconium to enhance the electrochemical performance of Ni-rich layered cathode materials. ACS Appl. Energy Mater. 3(12):11741–11751
Park HG, Min K, Park K (2022) A synergistic effect Na+ of and Al3+ dual doping on electrochemical performance and structural stability of LiNi0.88Co0.08Mn0.04O2 cathodes for Li-ion batteries. ACS Appl. Mater. Interfaces 14(4):5168–5176
Shi J, Ma Z, Han K, Wan Q, Wu D, Qu X, Li P (2022) Coupling novel Li7- TaO6 surface buffering with bulk Ta-doping to achieve long-life sulfide-based all-solid-state lithium batteries. J. Mater. Chem A 10(40):21336–21348
Zhang Y, Wang Z-B, Yu F-D, Que L-F, Wang M-J, Xia Y-F, Xue Y, Wu J (2017) Studies on stability and capacity for long-life cycle performance of Li (Ni0.5Co0.2Mn0.3)O2 by Mo modification for lithium-ion battery. J Power Sources 358:1–12
Wang J, Liu C, Wang Q, Xu G, Miao C, Xu M, Wang C, Xiao W (2022) Investigation of W6+-doped in high-nickel LiNi0.83Co0.11Mn0.06O2 cathode materials for high-performance lithium-ion batteries. J Colloid Interface Sci 628:338–349
Si Z, Shi B, Huang J, Yu Y, Han Y, Zhang J, Li W (2021) Titanium and fluorine synergetic modification improves the electrochemical performance of Li(Ni0.8Co0.1Mn0.1)O2. J. Mater. Chem. A 9(14):9354–9363
Kim H, Kim S-B, Park D-H, Park K-W (2020) Fluorine-doped LiNi0.8Mn0.1Co0.1O2 cathode for high-performance lithium-ion batteries. Energies 13(18):4808
Chen Z, Gong X, Zhu H, Cao K, Liu Q, Liu J, Li L and Duan J (2019) High performance and structural stability of K and Cl co-doped LiNi0.5Co0.2Mn0.3O2 cathode materials in 4.6 voltage. Frontiers in chemistry 6: 643
Do SJ, Santhoshkumar P, Kang SH, Prasanna K, Jo YN, Lee CW (2019) Al-doped Li[Ni0.78Co0.1Mn0.1Al0.02]O2 for high performance of lithium ion batteries. Ceram. Int. 45(6):6972–6977
Hou P, Li F, Sun Y, Pan M, Wang X, Shao M, Xu X (2018) Improving Li+ kinetics and structural stability of nickel-rich layered cathodes by heterogeneous inactive-Al3+ doping. ACS Sustain. Chem. Eng. 6(4):5653–5661
Jeong M, Kim H, Lee W, Ahn S-J, Lee E, Yoon W-S (2020) Stabilizing effects of Al-doping on Ni-rich LiNi0.80Co0.15Mn0.05O2 cathode for Li rechargeable batteries. J. Power Sources 474:228592
Li G, Zhou S, Wang P, Zhao J (2015) Halogen-doping in LiCoO2 cathode materials for Li-ion batteries: insights from ab initio calculations. RSC Adv 5(130):107326–107332
Kong F, Liang C, Longo RC, Yeon D-H, Zheng Y, Park J-H, Doo S-G, Cho K (2016) Conflicting roles of anion doping on the electrochemical performance of Li-ion battery cathode materials. Chem Mater 28(19):6942–6952
Zhou S, Wang G, Tang W, Xiao Y, Yan K (2018) Enhanced rate performance and high potential as well as decreased strain of LiNi0.6Co0.2Mn0.2O2 by facile fluorine modification. Electrochim Acta 261:565–577
Chen Z, Xu M, Zhu H, Xie T, Wang W, Zhao Q (2013) Enhanced electrochemical performance of polyacene coated LiMn2O3.95F0.05 for lithium ion batteries. Appl Surf Sci 286:177–183
Liao L, Wang X, Luo X, Wang X, Gamboa S, Sebastian P (2006) Synthesis and electrochemical properties of layered Li[Ni0.333Co0.333Mn0.293Al0.04]O2−zFz cathode materials prepared by the sol–gel method. J. Power Sources 160(1):657–661
Gong Z-L, Liu H-S, Guo X-J, Zhang Z-R, Yang Y (2004) Effects of preparation methods of LiNi0.8Co0.2O2 cathode materials on their morphology and electrochemical performance. J. Power Sources 136(1):139–144
Lee J-W, Lee J-H, Viet TT, Lee J-Y, Kim J-S, Lee C-H (2010) Synthesis of LiNi1/3Co1/3Mn1/3O2 cathode materials by using a supercritical water method in a batch reactor. Electrochim Acta 55(8):3015–3021
Croguennec L, Bains J, Bréger J, Tessier C, Biensan P, Levasseur S, Delmas C (2011) Effect of aluminum substitution on the structure, electrochemical performance and thermal stability of Li1+x(Ni0.40Mn0.40Co0.20−zAlz)1−xO2. J. Electrochem. Soc 158(6):664
Li K, Xue D (2006) Estimation of electronegativity values of elements in different valence states. J Phys Chem A 110(39):11332–11337
Kim S-B, Kim H, Park D-H, Kim J-H, Shin J-H, Jang J-S, Moon S-H, Choi J-H, Park K-W (2021) Li-ion diffusivity and electrochemical performance of Ni-rich cathode material doped with fluoride ions. J Power Sources 506:230219
Liu K, Zhang Q, Dai S, Li W, Liu X, Ding F, Zhang J (2018) Synergistic effect of F–doping and LiF coating on improving the high-voltage cycling stability and rate capacity LiNi0.5Co0.2Mn0.3O2 of cathode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 10(40):34153–34162
Yin S-C, Grondey H, Strobel P, Anne M, Nazar LF (2003) Electrochemical property: structure relationships in monoclinic Li3-yV2(PO4)3. J Am Chem Soc 125(34):10402–10411
Qiu L, Xiang W, Tian W, Xu C-L, Li Y-C, Wu Z-G, Chen T-R, Jia K, Wang D, He F-R (2019) Polyanion and cation co-doping stabilized Ni-rich Ni-Co-Al material as cathode with enhanced electrochemical performance for Li-ion battery. Nano Energy 63:103818
Ko G, Park S, Kim W, Kwon K (2022) Synergistic effect of Na and Al co-doping on the electrochemical properties Li[Ni0.8Mn0.1Co0.1]O2 of cathode materials for Li-ion batteries. J. Alloys Compd 925:166678
Huang Z, Wang Z, Zheng X, Guo H, Li X, Jing Q, Yang Z (2015) Effect of Mg doping on the structural and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials. Electrochim Acta 182:795–802
Hu G, Li L, Lu Y, Cao Y, Peng Z, Xue Z, Zhang Y, Fan J, Du K (2020) SrCO3 assisted synthesis of disk-like micron-sized monocrystalline LiNi0.5Co0.2Mn0.3O2 with Preferred (104) plane and its enhanced cycle performance. J. Electrochem. Soc 167(14):140505
Li Y-C, Xiang W, Wu Z-G, Xu C-L, Xu Y-D, Xiao Y, Yang Z-G, Wu C-J, Lv G-P, Guo X-D (2018) Construction of homogeneously Al3+ doped Ni rich Ni-Co-Mn cathode with high stable cycling performance and storage stability via scalable continuous precipitation. Electrochim Acta 291:84–94
Yang X, Tang Y, Shang G, Wu J, Lai Y, Li J, Qu Y, Zhang Z (2019) Enhanced cyclability and high-rate capability of LiNi0.88Co0.095Mn0.025O2 cathodes by homogeneous Al3+ doping. ACS Appl. Mater. Interfaces 11(35):32015–32024
Zhu Z, Liang Y, Hu H, Gao A, Meng T, Shu D, Yi F, Ling J (2021) Enhanced structural and electrochemical stability of LiNi0.83Co0.11Mn0.06O2 cathodes by zirconium and aluminum co-doping for lithium-ion battery. J. Power Sources 498:229857
Yue P, Wang Z, Guo H, Xiong X, Li X (2013) A low temperature fluorine substitution on the electrochemical performance of layered LiNi0.8Co0.1Mn0.1O2-zFz cathode materials. Electrochim Acta 92:1–8
Yang H, Wu HH, Ge M, Li L, Yuan Y, Yao Q, Chen J, Xia L, Zheng J, Chen Z (2019) Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium-ion batteries. Adv Func Mater 29(13):1808825
Li L, Chen Z, Zhang Q, Xu M, Zhou X, Zhu H, Zhang K (2015) A hydrolysis-hydrothermal route for the synthesis of ultrathin LiAlO2-inlaid LiNi0.5Co0.2Mn0.3O2 as a high-performance cathode material for lithium ion batteries. J. Mater. Chem. A 3(2):894–904
Choi W, Shin H-C, Kim JM, Choi J-Y, Yoon W-S (2020) Modeling and applications of electrochemical impedance spectroscopy (EIS) for lithium-ion batteries. J Electrochem Sci Technol 11(1):1–13
He H, Cong H, Sun Y, Zan L, Zhang Y (2017) Spinel-layered integrate structured nanorods with both high capacity and superior high-rate capability as cathode material for lithium-ion batteries. Nano Res 10:556–569
Zhao Z, Liu Y, Luo B, Shen J, Wang C, Zhang J, Cheng L, Xiao Z, Ming L, Zhang B (2021) Slower capacity/voltage degradation of surface LiNi0.92Co0.05Mn0.03O2 engineered cathode for lithium-ion batteries. Applied surface science 570:151017
Acknowledgements
The authors would like to appreciate the help of XPS tests from Shiyanjia Lab (www.shiyanjia.com). And this research was provided technical support by “Ceshigo Research Service Agency for EBSD (www.ceshigo.com).”
Funding
This study was financially supported by the National Natural Science Foundation of China (Grant No. 51874358 and 51772333) and supported by Changsha Municipal Natural Science Foundation (kq2014128).
Author information
Authors and Affiliations
Contributions
Zhongdong Peng: resources, investigation, same contribution as Qiuming Yan. Qiuming Yan: methodology, investigation, writing—review and editing. Ke Du: supervision, resources. Guorong Hu: supervision, resources. Zhongyuan Luo: review. Zijun Fang: review&TEM test. Zhiying Li: technical guidance. Xin Wang: technical guidance. Qinglai Jiang (second corresponding author): resources, technical guidance, review and editing. Yanbing Cao (first corresponding author): supervision, data curation and review, resources.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, Z., Yan, Q., Du, K. et al. Enhancing the electrochemical properties of LiNi0.92Co0.05Mn0.03O2 cathode material via co-doping aluminium and fluorine for high-energy lithium-ion batteries. Ionics 29, 3013–3025 (2023). https://doi.org/10.1007/s11581-023-05015-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05015-w