Abstract
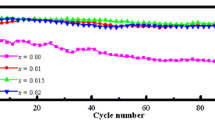
Lithium-rich manganese-based material is one of the most promising cathode materials for Li ion battery due to its low cost and high specific capacity. However, the irreversible evolution of oxygen and migration of transition metals (TM) restricts its widely practical application. In this paper, F-doped Li1.2[Mn0.54Ni0.13Co0.13]O2 was prepared by co-precipitation and high temperature solid-state reaction method. The effects of F-doping on the electrochemical properties of Li1.2[Mn0.54Ni0.13Co0.13]O2 during the charge–discharge procedure were investigated. Results show that Li1.2[Mn0.54Ni0.13Co0.13]O1.95F0.05 sample has the excellent cycling stability; the discharge-specific capacity reaches 182.9 mAh g−1 after 200 cycles, with a capacity retention rate of 87.9%, which was much higher than that of the original sample (133.2 mAh g−1 and 77.5%). The relevant properties proved that F-doping can inhibit the irreversible evolution of oxygen, alleviate the structural degradation of the material, and further alleviate the dissolution of transition metals by suppressing the interfacial side reactions. This result provides reference for the practical application of Li-rich cathode materials.









Similar content being viewed by others
References
Zhang R, Huang X, Wang D, Hoang TKA, Yang Y, Duan X, Chen P, Qin LC, Wen G (2018) Single-phase mixed transition metal carbonate encapsulated by graphene: facile synthesis and improved lithium storage properties. Adv Func Mater 28(10):1705817
Wang D, Qi S, Qiu Y, Zhang R, Zhang Q, Liu S, Zhang C, Chen Z, Pan H, Cao J, Wen G (2020) High-yield production of non-layered 2D carbon complexes: thickness manipulation and carbon nanotube branches for enhanced lithium storage properties. J Energy Chem 59:19–29
Shi SJ, Lou ZR, Xia TF, Wang XL, Gu CD, Tu JP (2014) Hollow Li1.2Mn0.5Co0.25Ni0.05O2 microcube prepared by binary template as a cathode material for lithium ion batteries. J Power Sources 257:198–204
He W, Guo W, Wu H, Lin L, Liu Q, Han X, Xie Q, Liu P, Zheng H, Wang L, Yu X, Peng DL (2021) Challenges and recent advances in high capacity Li-rich cathode materials for high energy density lithium-ion batteries. Adv Mater 33:2005937
Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Benedek R, Hackney S (2007) Li2MnO3-stabilized LiMO2 (M= Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem 17:3112–3125
Ku K, Hong J, Kim H, Park H, Seong WM, Jung SK, Yoon G, Park KY, Kim H, Kang K (2018) Suppression of voltage decay through manganese deactivation and nickel redox buffering in high-energy layered lithium-rich electrodes. Adv Energy Mater 8:1800606
Koga H, Croguennec L, Ménétrier M, Douhil K, Belin S, Bourgeois L, Suard E, Weill F, Delmas C (2013) Reversible oxygen participation to the redox processes revealed for Li1.20Mn0.54Co0.13Ni0.13O2. J Electrochem Soc 160:A786
Xu B, Fell CR, Chi M, Meng YS (2011) Identifying surface structural changes in layered Li-excess nickel manganeseoxides in high voltage lithium ion batteries: a joint experimental and theoretical study. Energy Environ Sci 4:2223–2233
Lin T, Schulli TU, Hu Y, Zhu X, Gu Q, Luo B, Cowie B, Wang L (2020) Faster activation and slower capacity/voltage fading: a bifunctional urea treatment on lithium-rich cathode materials. Adv Func Mater 30:1909192
Hong J, Lim HD, Lee M, Kim SW, Kim H, Oh ST, Chung GC, Kang K (2012) Critical role of oxygen evolved from layered Li–excess metal oxides in lithium rechargeable batteries. Chem Mater 24:2692–2697
Hu E, Yu X, Lin R, Bi X, Lu J, Bak S, Nam KW, Xin HL, Jaye C, Fischer DA, Amine K, Yang XQ (2018) Evolution of redox couples in Li and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat Energy 3:690–698
Hekmatfar M, Kazzazi A, Eshetu GG, Hasa I, Passerini S (2019) Understanding the electrode/electrolyte interface layer on the Li-rich nickel manganese cobalt layered oxide cathode by XPS. ACS Appl Mater Interfaces 11:43166–43179
Yan P, Zheng J, Chen T, Luo L, Jiang Y, Wang K, Sui M, Zhang JG, Zhang S, Wang C (2018) Coupling of electrochemically triggered thermal and mechanical effects to aggravate failure in a layered cathode. Nat Commu 9:1–8
Hu Y, Qin Z, Pei J, Cong B, Yang X, Chen G (2020) Reduced Li/Ni disorder degree of Na-doped Li-rich layered oxide for cathode material: experimental and calculations. ChemElectroChem 7:246–251
Sun YX, Zhang LJ, Dong SD, Zeng JB, Shen Y, Li X, Ren XF, Ma LX, Hai CX, Zhou Y (2022) Improving the electrochemical performances of Li-rich Li1.2Ni0.13Co0.13Mn0.54O2 through cooperative doping of Na+ and Mg2+. Electrochim Acta 414:140169
Lai XW, Hu GR, Peng ZD, Cao YB, Wang WG, Du K (2022) Cerium-doped lithium-rich Li1.2Mn0.56Ni0.11Co0.13O2 as cathode with high performance for lithium-ion batteries. Ionics:1–12
Huang X, Zhang Z, He J, Bai Z, Lu L, Li J (2021) Effects of chromium/fluorine co-doping on the electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 cathode material for lithium-ion batteries. J Mater Sci 56:9836–9851
Zheng J, Wu X, Yang Y (2013) Improved electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material by fluorine incorporation. Electrochim Acta 105:200–208
Wang B, Cui J, Li Z, Wang H, Zhang D, Wang Q, Sun H, Hu Z (2022) Surface F-doping for stable structure and high electrochemical performance of Li-rich Mn-based cathode materials. J Alloys Comp 929:167304
Guo WB, Zhang CY, Zhang YG, Lin L, He W, Xie QS, Sa BS, Wang LS, Peng DL (2021) A universal strategy toward the precise regulation of initial coulombic efficiency of Li-rich Mn-based cathode materials. Adv Mater 33:2103173
Rozier P, Tarascon JM (2015) Li-rich layered oxide cathodes for next-generation Li-ion batteries: chances and challenges. J Electrochem Soc 162:A2490
Dahiya PP, Ghanty C, Sahoo K, Basu S, Majumder SB (2018) Suppression of voltage decay and improvement in electrochemical performance by zirconium doping in Li-rich cathode materials for Li-ion batteries. J Electrochem Soc 165(13):A3114
Fell CR, Qian D, Carroll KJ, Chi M, Jones JL, Meng YS (2013) Correlation between oxygen vacancy, microstrain, and cation distribution in lithium-excess layered oxides during the first electrochemical cycle. Chem Mater 25:1621–1629
Song B, Lai MO, Lu L (2012) Influence of Ru substitution on Li-rich 0.55Li2MnO3·0.45 LiNi1/3Co1/3Mn1/3O2 cathode for Li-ion batteries. Electrochim Acta 80:187–195
Ceder G (2010) Opportunities and challenges for first-principles materials design and applications to Li battery materials. MRS bull 35:693–701
Song B, Lai MO, Liu Z, Liu H, Lu L (2013) Graphene-based surface modification on layered Li-rich cathode for high-performance Li-ion batteries†. J Mater Chem A 1(34):9954–9965
Zou Y, Yang X, Lv C, Liu T, Xia Y, Shang L, Waterhouse GI, Yang D, Zhang T (2017) Multishelled Ni-rich Li(NixCoyMnz)O2 hollow fibers with low cation mixing as high-performance cathode materials for Li-ion batteries. Adv Sci 4:1600262
Li L, Wang L, Zhang X, Xue Q, Wei L, Wu F, Chen R (2017) 3D reticular Li1. 2Ni0.2Mn0.6O2 cathode material for lithium-ion batteries. ACS Appl Mater Interfaces 9:1516–1523
Peng Z, Mu K, Cao Y, Xu L, Du K, Hu G (2019) Enhanced electrochemical performance of layered Li-rich cathode materials for lithium ion batteries via aluminum and boron dual-doping. Ceram Int 45:4184–4192
Gao XG, Li SH, Zhang HY, Zhang S, Chang SL, Li HX, Li S, Lai YQ, Zhang Z (2022) Constructing a robust integrated surface structure for enhancing the performance of Li-rich Mn-based oxides cathodes. Mater Today Energy 30:101152
Lee S, Jin W, Kim SH, Joo SH, Nam G, Oh P, Kim KY, Kwak SK, Cho J (2019) Oxygen vacancy diffusion and condensation in lithium-ion battery cathode materials. Angew Chem Int Edit 58(31):10478–10485
Ma Q, Chen Z, Zhong S, Meng J, Lai F, Li Z, Cheng C, Zhang L, Liu T (2020) Na-substitution induced oxygen vacancy achieving high transition metal capacity in commercial Li-rich cathode. Nano Energy 81:105622
Zheng J, Gu M, Xiao J, Polzin BJ, Yan P, Chen X, Wang C, Zhang JG (2014) Functioning mechanism of AlF3 coating on the Li and Mn-rich cathode materials. Chem Mater 26(22):6320–6327
Gu M, Belharouak I, Zheng J, Wu H, Xiao J, Genc A, Amine K, Thevuthasan S, Baer DR, Zhang J-G (2013) Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 7:760–767
Si M, Wang D, Zhao R, Pan D, Zhang C, Yu C, Lu X, Zhao H, Bai Y (2019) Local electric-field-driven fast Li diffusion kinetics at the piezoelectric LiTaO3 modified Li-rich cathode-electrolyte interphase. Adv Sci 7(3):1902538
Chernyavsky V, Kim A, Koshtyal Y, Rumyantsev A, Popovich A, Maximov MY (2022) Structural features of complete and partial activation of Li-rich cathodes studied by in-situ XRD. Electrochimica Acta 414:140237
Li X, Qiao Y, Guo S, Xu Z, Zhu H, Zhang X, Yuan Y, He P, Ishida M, Zhou H (2018) Direct visualization of the reversible O2−/O− redox process in Li-rich cathode materials. Adv Mater 30(14):1705197
Zheng H, Zhang C, Zhang Y, Lin L, Liu P, Wang L, Wei Q, Lin J, Sa B, Xie Q, Peng DL (2021) Manipulating the local electronic structure in Li-rich layered cathode towards superior electrochemical performance. Adv Func Mater 31(30):2100783
Zhang XD, Shi JL, Liang JY, Yin YX, Zhang JN, Yu XQ, Guo YG (2018) Suppressing surface lattice oxygen release of Li-rich cathode materials via heterostructured spinel Li4Mn5O12 coating. Adv Mater 30(29):1801751
Zhu W, Tai Z, Shu C, Chong S, Guo S, Ji L, Chen Y, Liu Y (2020) The superior electrochemical performance of a Li-rich layered cathode material with Li-rich spinel Li4Mn5O12 and MgF2 double surface modifications. J Mater Chem A 8:7991–8001
Wang Y, Liu F, Fan G, Qiu X, Liu J, Yan Z, Zhang K, Cheng F, Chen J (2021) Electroless formation of a fluorinated Li/Na hybrid interphase for robust lithium anodes. J Am Chem Soc 143:2829–2837
Zheng J, Wu X, Yang Y (2013) Improved electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material by fluorine incorporation. Electrochimica Acta 105:200–208
Chen L, Fan X, Hu E, Ji X, Chen J, Hou S, Deng T, Li J, Su D, Yang X (2019) Achieving high energy density through increasing the output voltage: a highly reversible 5.3 V battery. Chem 5:896–912
Chen J, Zou G, Deng W, Huang Z, Gao X, Liu C, Yin S, Liu H, Deng X, Tian Y (2020) Pseudo-bonding and electric-field harmony for Li-rich Mn-based oxide cathode. Adv Funct Mater 30:2004302
Fan X, Hu G, Zhang B, Ou X, Zhang J, Zhao W, Jia H, Zou L, Li P, Yang Y (2020) Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 70:104450
Ming Y, Xiang W, Qiu L, Hua WB, Li R, Wu ZG, Xu CL, Li YC, Wang D, Chen YX (2020) Dual elements coupling effect induced modification from the surface into the bulk lattice for Ni-rich cathodes with suppressed capacity and voltage decay. ACS Appl Mater 12:8146–8156
Kim K, Hwang D, Kim S, Park SO, Cha H, Lee YS, Cho J, Kwak SK, Choi NS (2020) Cyclic aminosilane-based additive ensuring stable electrode–electrolyte interfaces in Li-ion batteries. Adv Energy Mater 10:2000012
He W, Qian J, Cao Y, Ai X, Yang H (2012) Improved electrochemical performances of nanocrystalline Li [Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for Li-ion batteries. RSC Adv 2:3423–3429
Wu B, Yang X, Jiang X, Zhang Y, Shu H, Gao P, Liu L, Wang X (2018) Synchronous tailoring surface structure and chemical composition of Li-rich–layered oxide for high-energy lithium-ion batteries. Adv Func Mater 28:1803392
Funding
This research is funded by the Natural Science Foundation of China (No. 51504111 and 51564029), China Postdoctoral Science Foundation (2018M633418) and Analysis and Testing Foundation of Kunming University of Science and Technology (2021M20202202075).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, C., Zhang, P., Huang, H. et al. Unveiling the enhancing mechanism of cycling stability of Li1.2Mn0.54Ni0.13Co0.13O2-xFx cathode materials. Ionics 29, 2573–2586 (2023). https://doi.org/10.1007/s11581-023-05003-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05003-0