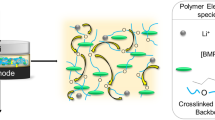
Abstract
In this study, zwitterionic poly(ionic liquid)-based polymer electrolytes with a network structure are prepared by ultraviolet-irradiation radical polymerization of poly(ethylene glycol) methyl ether acrylate (PEGMEA) and zwitterionic liquids, and poly(ethylene glycol) diacrylate is used as cross-linker. The cross-linked structure endows the electrolytes with high thermal stability and excellent dimensional stability. The abundant ether-oxygen group of PEGMEA provides sufficient sites for the transport of Li+ and a high ionic conductivity of 0.20 × 10−3 S cm−1. The electrolyte shows a high lithium-ion transference number of 0.78, owing to the excellent dissociation ability of zwitterionic liquids towards lithium salts. The lithium symmetric battery can maintain a voltage polarization of 150 mV at 0.1 mA cm−2 over 600 h. The Li/LiFePO4 battery shows a discharge capacity of 122 mAh g−1, a capacity retention of 90.0% after 100 cycles, and an average Coulombic efficiency exceeding 99% at 0.1 C, demonstrating strong application potential in lithium-ion batteries.








Similar content being viewed by others
References
Goodenough JB, Park K-S (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135(4):1167–1176. https://doi.org/10.1021/ja3091438
Chu S, Cui Y, Liu N (2017) The path towards sustainable energy. Nat Mater 16(1):16–22. https://doi.org/10.1038/nmat4834
Xie J, Lu Y-C (2020) A retrospective on lithium-ion batteries. Nat Commun 11(1):2499. https://doi.org/10.1038/s41467-020-16259-9
Niu H, Zhang N, Ding M, Li X, Su X, Guo X, Guan P, Hu X (2022) D-endritic mesoporous silica composite ionic liquid gel polymer electrolyte improves the performance of lithium battery. Ionics 28(8):3761–3775. https://doi.org/10.1007/s11581-022-04609-0
Niu H, Wang L, Guan P, Zhang N, Yan C, Ding M, Guo X, Huang T, Hu X (2021) Recent advances in application of ionic liquids in electrolyte of lithium ion batteries. J Energy Storage 40:102659. https://doi.org/10.1016/j.est.2021.102659
Feng X, Ouyang M, Liu X, Lu L, Xia Y, He X (2018) Thermal runaway mechanism of lithium ion battery for electric vehicles: a review. Energy Stor Mater 10:246–267. https://doi.org/10.1016/j.ensm.2017.05.013
He X, Schmohl S, Wiemhöfer HD (2019) Comparative study of interfacialbehavior for polyphosphazene based polymer electrolytes and LiPF6 in EC/DMC against lithium metal anodes. Polym Test 76:505–512. https://doi.org/10.1016/j.polymertesting.2019.04.012
Deng K, Zeng Q, Wang D, Liu Z, Wang G, Qiu Z, Zhang Y, Xiao M, Meng Y (2020) Nonflammable organic electrolytes for high-safety lithium-ionbatteries. Energy Stor Mater 32:425–447. https://doi.org/10.1016/j.ensm.2020.07.018
Bi Z, Mu S, Zhao N, Sun W, Huang W, Guo X (2021) Cathode supported solid lithium batteries enabling high energy density and stable cyclability. Energy Stor Mater 35:512–519. https://doi.org/10.1016/j.ensm.2020.11.038
Yu Y, Liu Y, Xie J (2021) Building better Li metal anodes in liquid electrolyte: challenges and progress. ACS Appl Mater Interfaces 13(1):18–33. https://doi.org/10.1021/acsami.0c17302
Wang J, Yamada Y, Sodeyama K, Watanabe E, Takada K, Tateyama Y, Yamada A (2018) Fire-extinguishing organic electrolytes for safe batteries. Nat Energy 3(1):22–29. https://doi.org/10.1038/s41560-017-0033-8
Jiang L, Wang Q, Li K, Ping P, Jiang L, Sun J (2018) A self-cooling and flame-retardant electrolyte for safer lithium ion batteries. Sustain Energy Fuels 2(6):1323–1331. https://doi.org/10.1039/C8SE00111A
Deb D, Bose P, Bhattacharya S (2021) Gel-polymer electrolytes plasticized with pyrrolidinium-based ionanofluid for lithium battery applications. Ionics 27(1):123–136. https://doi.org/10.1007/s11581-020-03807-y
Grande L, von Zamory J, Koch SL, Kalhoff J, Paillard E, Passerini S (2015) Homogeneous lithium electrodeposition with pyrrolidinium-based ionic liquid electrolytes. ACS Appl Mater Interfaces 7(10):5950–5958. https://doi.org/10.1021/acsami.5b00209
Moreno JS, Deguchi Y, Panero S, Scrosati B, Ohno H, Simonetti E, Appetecchi GB (2016) N-Alkyl-N-ethylpyrrolidinium cation-based ionic liquid electrolytes for safer lithium battery systems. Electrochim Acta 191:624–630. https://doi.org/10.1016/j.electacta.2016.01.119
Wang H, Sheng L, Yasin G, Wang L, Xu H, He X (2020) Reviewing the current status and development of polymer electrolytes for solid-state lithium batteries. Energy Stor Mater 33:188–215. https://doi.org/10.1016/j.ensm.2020.08.014
Ding W-Q, Lv F, Xu N, Wu M-T, Liu J, Gao X-P (2021) Polyethylene oxide-based solid-state composite polymer electrolytes for rechargeable lithium batteries. ACS Appl Energy Mater 4(5):4581–4601. https://doi.org/10.1021/acsaem.1c00216
Alzate-Carvajal N, Rousselot S, Storelli A, Gelinas B, Zhang X, Malveau C, Rochefort D, Dollé M (2022) A comparative study on the influence of the polymeric host for the operation of all-solid-state batteries at different temperatures. J Power Sources 535:231382. https://doi.org/10.1016/j.jpowsour.2022.231382
Fu C, Homann G, Grissa R, Rentsch D, Zhao W, Gouveia T, Falgayrat A,Lin R, Fantini S, Battaglia C (2022)A polymerized-ionic-liquid-based polymer electrolyte with high oxidative stability for 4 and 5 V class solid-state lithium metal batteries. Adv Energy Mater 12(27):2200412. https://doi.org/10.1002/aenm.202200412.
Jia M, Wen P, Wang Z, Zhao Y, Liu Y, Lin J, Chen M, Lin X (2021) Fluorinated bifunctional solid polymer electrolyte synthesized under visible light for stable lithium deposition and dendrite-free all-solid-state batteries. Adv Funct Mater 31(27):2101736. https://doi.org/10.1002/adfm.202101736
Dubey BP, Sahoo A, Thangadurai V, Sharma Y (2022) Fabrication of ultra-thin, flexible, dendrite-free, robust and nanostructured solid electrolyte membranes for solid-state Li-batteries. J Mater Chem A 10(22):12196–12212. https://doi.org/10.1039/D2TA01412B
Yang Z, Yuan H, Zhou C, Wu Y, Tang W, Sang S, Liu H (2020) Facile interfacial adhesion enabled LATP-based solid-state lithium metal battery. Chem Eng J 392:123650. https://doi.org/10.1016/j.cej.2019.123650
Huang X, Wu J, Wang X, Tian Y, Zhang F, Yang M, Xu B, Wu B, Liu X, Li H (2021) In situ synthesis of a Li6.4La3Zr1.4Ta0.6O12/poly(vinylene carbonate) hybrid solid-state electrolyte with enhanced ionic conductivity and stability. ACS Appl Energy Mater 4(9):9368–9375. https://doi.org/10.1021/acsaem.1c01570
Zhang X, Sun Y, Ma C, Guo N, Fan H, Liu J, Xie H (2022) Li6.4La3Zr1.4Ta0.6O12 reinforced polystyrene-poly(ethylene oxide)-poly(propylene oxide)-po-ly(ethylene oxide)-polystyrene pentablock copolymer-based composite solid electrolytes for solid-state lithium metal batteries. J Power Sources 542:231797. https://doi.org/10.1016/j.jpowsour.2022.231797
Li W, Zhu X, Zhou N, Yang Y, Li R, Wang C, Fang Z, Ma X, Zhao W, Fu X, Yan W (2021) Helical polyurethane-initiated unique microphase separation architecture for highly efficient lithium transfer and battery performance of a poly(ethylene oxide)-based all-solid-state electrolyte. ACS Appl Energy Mater 4(5):4772–4785. https://doi.org/10.1021/acsaem.1c00358
Zhou F, Liao H, Zhang Z (2020) Mechanical strong polymer cross-linking PVDF nanofiber electrolyte for lithium-ion batteries. Ionics 26(8):3893–3900. https://doi.org/10.1007/s11581-020-03549-x
Yap YL, You AH, Teo LL (2019) Preparation and characterization studies of PMMA–PEO-blend solid polymer electrolytes with SiO2 filler and plasticizer for lithium ion battery. Ionics 25(7):3087–3098. https://doi.org/10.1007/s11581-019-02842-8
Ma Y, Wan J, Yang Y, Ye Y, Xiao X, Boyle DT, Burke W, Huang Z, Chen H, Cui Y, Yu Z, Oyakhire ST, Cui Y (2022) Scalable, ultrathin, andhigh-temperature-resistant solid polymer electrolytes for energy-dense lithiummetal batteries. Adv Energy Mater 12(15):2103720. https://doi.org/10.1002/aenm.202103720
Qi H, Ren Y, Guo S, Wang Y, Li S, Hu Y, Yan F (2020) High-voltage resistant ionic liquids for lithium-ion batteries. ACS Appl Mater Interfaces 12(1):591–600. https://doi.org/10.1021/acsami.9b16786
Huang M, Kan L, Zhao W, Wang Y, Xiong Y, Shan W, Lou Z (2021) Highly efficient and selective capture of TcO4- or ReO4- by imidazolium-basedionic liquid polymers. Chem Eng J 421:127763. https://doi.org/10.1016/j.cej.2020.127763
Tang X, Lv S, Jiang K, Zhou G, Liu X (2022) Recent development of ionic liquid-based electrolytes in lithium-ion batteries. J Power Sources 542:231792. https://doi.org/10.1016/j.jpowsour.2022.231792
Wang Z, Zhang F, Sun Y, Zheng L, Shen Y, Fu D, Li W, Pan A, Wang L, Xu J, Hu J, Wu X (2021) Intrinsically nonflammable ionic liquid-based localized highly concentrated electrolytes enable high-performance Li-metal batteries. Adv Energy Mater 11(17):2003752. https://doi.org/10.1002/aenm.202003752
Tsurumaki A, Kagimoto J, Ohno H (2011) Properties of polymer electrolytes composed of poly(ethylene oxide) and ionic liquids according to hard and soft acids and bases theory. Polym Adv Technol 22(8):1223–1228. https://doi.org/10.1002/pat.1931
Sen S, Goodwin SE, Barbará PV, Rance GA, Wales D, Cameron JM, Sans V, Mamlouk M, Scott K, Walsh DA (2021) Gel-polymer electrolytes based on poly(ionic liquid)/ionic liquid networks. ACS Appl Polym Mater 3(1):200–208. https://doi.org/10.1021/acsapm.0c01042
Li B, Zhao S, Zhu J, Ge S, Xing K, Sokolov AP, Saito T, Cao P-F (2021) Rational polymer design of stretchable poly(ionic liquid) membranes for dual applications. Macromolecules 54(2):896–905. https://doi.org/10.1021/acs.macromol.0c02335
Wang Z, Zheng W, Sun W, Zhao L, Yuan W (2021) Covalent organic frameworks-enhanced ionic conductivity of polymeric ionic liquid-based ionic gel electrolyte for lithium metal battery. ACS Appl Energy Mater 4(3):2808–2819. https://doi.org/10.1021/acsaem.0c03229
Zhou N, Wang Y, Zhou Y, Shen J, Zhou Y, Yang Y (2019) Star-shaped multi-arm polymeric ionic liquid based on tetraalkylammonium cation as high performance gel electrolyte for lithium metal batteries. Electrochim Acta 301:284–293. https://doi.org/10.1016/j.electacta.2019.01.143
Zhang F, Sun Y, Wang Z, Fu D, Li J, Hu J, Xu J, Wu X (2020) Highly conductive polymeric ionic liquid electrolytes for ambient-temperature solid-state lithium batteries. ACS Appl Mater Interfaces 12(21):23774–23780. https://doi.org/10.1021/acsami.9b22945
Zhu X, Fang Z, Deng Q, Zhou Y, Fu X, Wu L, Yan W, Yang Y (2022) Poly(ionic liquid)@PEGMA block polymer initiated microphase separation architecture in poly(ethylene oxide)-based solid-state polymer electrolyte for flexible and self-healing lithium batteries. ACS Sustain Chem Eng 10(13):4173–4185. https://doi.org/10.1021/acssuschemeng.1c08306
Guo C, Liu D, Wei J, Chen F (2021) Polymerized ionic networks solid electrolyte with high ionic conductivity for lithium batteries. Ind Eng Chem Res 60(12):4630–4638. https://doi.org/10.1021/acs.iecr.0c05519
Sha Y, Yu T, Dong T, Wu X-l, Tao H, Zhang H (2021) In situ network electrolyte based on a functional polymerized ionic liquid with high conductivity toward lithium metal batteries. ACS Appl Energy Mater 4(12):14755–14765. https://doi.org/10.1021/acsaem.1c03443
Qin H, Panzer MJ (2020) Zwitterionic copolymer-supported ionogel electrolytes featuring a sodium salt/ionic liquid solution. Chem Mater 32(18):7951–7957. https://doi.org/10.1021/acs.chemmater.0c02820
Sun R, Agrawal M, Neyerlin KC, Snyder JD, Elabd YA (2022) Proton conducting sulfonated poly(ionic liquid) block copolymers. Macromolecules 55(15):6716–6729. https://doi.org/10.1021/acs.macromol.2c00468
Gu Y, Yang L, Luo S, Zhao E, Saito N (2022) A non-flammable, flexible and UV-cured gel polymer electrolyte with crosslinked polymer network fordendrite-suppressing lithium metal batteries. Ionics 28(8):3743–3759. https://doi.org/10.1007/s11581-022-04621-4
Tiyapiboonchaiya C, Pringle JM, MacFarlane DR, Forsyth M, Sun J (2003) Polyelectrolyte-in-ionic-liquid electrolytes. Macromol Chem Phys 204(17):2147–2154. https://doi.org/10.1002/macp.200350073
Lu F, Li G, Yu Y, Gao X, Zheng L, Chen Z (2020) Zwitterionic impetus on single lithium-ion conduction in solid polymer electrolyte for all-solid-state lithium-ion batteries. Chem Eng J 384:123237. https://doi.org/10.1016/j.cej.2019.123237
Wang C, Chen P, Wang Y, Chen T, Liu M, Zhang M, Fu Y, Xu J, Fu J (2022) Synergistic cation-anion regulation of polysulfides by zwitterionic polymer binder for lithium-sulfur batteries. Adv Funct Mater 32(34):2204451. https://doi.org/10.1002/adfm.202204451
Chen L, Fu J, Lu Q, Shi L, Li M, Dong L, Xu Y, Jia R (2019) Cross-linked polymeric ionic liquids ion gel electrolytes by in situ radical polymerization. Chem Eng J 378:122245. https://doi.org/10.1016/j.cej.2019.122245
Chen H, Tu H, Hu C, Liu Y, Dong D, Sun Y, Dai Y, Wang S, Qian H, Lin Z, Chen L (2018) Cationic covalent organic framework nanosheets for fast Li-ion conduction. J Am Chem Soc 140(3):896–899. https://doi.org/10.1021/jacs.7b12292
Nava DP, Guzmán G, Vazquez-Arenas J, Cardoso J, Gomez B, Gonzalez I (2016) An experimental and theoretical correlation to account for the effect of LiPF6 concentration on the ionic conductivity of poly(poly (ethylene glycol) methacrylate). Solid State Ion 290:98–107. https://doi.org/10.1016/j.ssi.2016.03.020
Zamory J, Giffin GA, Jeremias S, Castiglione F, Mele A, Paillard E, Passerini S (2016) Influence of oligo(ethylene oxide) substituents on pyrrolidinium-based ionic liquid properties, Li+ solvation and transport. Phys Chem Chem Phys 18(31):21539–21547. https://doi.org/10.1039/C6CP02092E
Wang Z, Guo Q, Jiang R, Deng S, Ma J, Cui P, Yao X (2022) Porous poly(vinylidene fluoride) supported three-dimensional poly(ethylene glycol) thin solid polymer electrolyte for flexible high temperature all-solid-state lithium metal batteries. Chem Eng J 435:135106. https://doi.org/10.1016/j.cej.2022.135106
Liu X, Liu J, Lin B, Chu F, Ren Y (2022) PVDF-HFP-based composite electrolyte membranes having high conductivity and lithium-ion transference number for lithium metal batteries. ACS Appl Energy Mater 5(1):1031–1040. https://doi.org/10.1021/acsaem.1c03417
Chen Z, Steinle D, Nguyen HD, Kim JK, Mayer A, Shi JL, Paillard E, Iojoiu C, Passerini S, Bresser D (2020) High-energy lithium batteries based on single-ion conducting polymer electrolytes and Li[Ni0.8Co0.1Mn0.1]O2 cathodes. Nano Energy 77:105129. https://doi.org/10.1016/j.nanoen.2020.105129
Liang L, Chen X, Yuan W, Chen H, Liao H, Zhang Y (2021) Highly conductive, flexible, and nonflammable double-network poly(ionic liquid)-based ionogel electrolyte for flexible lithium-ion batteries. ACS Appl Mater Interfaces 13(21):25410–25420. https://doi.org/10.1021/acsami.1c06077
Zhang J, Wang S, Han D, Xiao M, Sun L, Meng Y (2020) Lithium (4-styrenesulfonyl) (trifluoromethanesulfonyl) imide based single-ion polymer electrolyte with superior battery performance. Energy Stor Mater 24:579–587. https://doi.org/10.1016/j.ensm.2019.06.029
Li Y, Sun Z, Shi L, Lu S, Sun Z, Shi Y, Wu H, Zhang Y, Ding S (2019) Poly(ionic liquid)-polyethylene oxide semi-interpenetrating polymer network solid electrolyte for safe lithium metal batteries. Chem Eng J 375:121925. https://doi.org/10.1016/j.cej.2019.121925
Hu Z, Chen J, Guo Y, Zhu J, Qu X, Niu W, Liu X (2020) Fire-resistant, high-performance gel polymer electrolytes derived from poly(ionic liquid)/P(VDF-HFP) composite membranes for lithium ion batteries. J Membr Sci 599:117827. https://doi.org/10.1016/j.memsci.2020.117827
Funding
This work was supported by National Natural Science Foundation of China (No.51973022), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX223037), and Qing Lan project of Jiangsu province.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1.
(DOC 7836 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Xu, Y., Xu, F. et al. Zwitterionic poly(ionic liquids)-based polymer electrolytes for Lithium-ion batteries applications. Ionics 29, 2249–2259 (2023). https://doi.org/10.1007/s11581-023-04978-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04978-0