Abstract
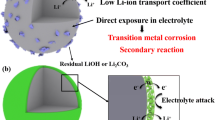
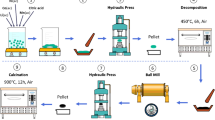
Layered lithium-rich oxide materials are regarded as one of the most promising cathode materials. However, inferior cycling stability and poor rate performance hinder their practical application prospect. In this study, Li3PO4-coated Li1.2Ni0.2Mn0.6O2 cathode materials have been synthesized by sol–gel method together with a facile liquid-evaporation process. The results suggested that the Li3PO4 coating layer, which could not only facilitate the lithium-ion diffusion rate and accelerate the diffusion kinetics but also act as a protective layer to protect it from corrosion by HF and other side reactions. Density functional theory (DFT) calculations confirmed the essence effect on lithium-ion diffusion coefficient and electronic conductivity. After modifying with an appropriate amount of Li3PO4, the Li-rich layered oxides showed enhanced electrochemical performance. Especially, the capacity retention of 5 wt% Li3PO4-coated Li1.2Ni0.2Mn0.6O2 was significantly enhanced from 17.7% of the bare Li1.2Ni0.2Mn0.6O2 to 73.8%. In terms of rate capabilities, 5 wt% Li3PO4-coated Li1.2Ni0.2Mn0.6O2 retained capacities of 181.0, 165.9, 128.8, and 107.8 mAh g−1, while the bare Li1.2Ni0.2Mn0.6O2 were only 137.4, 109.3, 75.6, and 45.9 mAh g−1, respectively, at rates of 0.5 C, 1 C, 2 C, and 5 C. Our research findings show that coating with an appropriate amount of lithium-ion conductor material is one of the effective measures to obtain improved performance of Li-rich and Mn-rich layered oxide materials.










Similar content being viewed by others
Data Availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information files.
References
Harper G, Sommerville R, Kendrick E, Driscoll L, Slater P, Stolkin R, Walton A, Christensen P, Heidrich O, Lambert S, Abbott A, Ryder KS, Gaines L, Anderson P (2019) Recycling lithium-ion batteries from electric vehicles. Nature 575(7781):75–86
Pender JP, Jha G, Youn DH, Ziegler JM, Andoni I, Choi EJ, Heller A, Dunn BS, Weiss PS, Penner RM, Mullins CB (2020) Electrode degradation in lithium-ion batteries. ACS Nano 14(2):1243–1295
Lee W, Muhammad S, Sergey C, Lee H, Yoon J, Kang YM, Yoon WS (2020) Advances in the cathode materials for lithium rechargeable batteries. Angewandte Chemie-Int Ed 59(7):2578–2605
Manthiram A (2020) A reflection on lithium-ion battery cathode chemistry. Nat Commun 11(1):1550
Yin C, Zhou H, Yang Z, Li J (2018) Synthesis and electrochemical properties of LiNi0.5Mn1.5O4 for Li-ion batteries by the metal-organic framework method. ACS Appl Mater Interfaces 10:13625–13634
Yin C, Bao Z, Tan H, Zhou H, Li J (2019) Metal-organic framework-mediated syntheisi of LiNi0.5 Mn1.5 O4: tuning the Mn3+ content and electrochemical performance by organic ligands. Chem Eng J 372:408–419
Liu W-J, Sun X-Z, Zhang X, Li C, Wang K, Wen W, Ma Y-W (2021) Structural evolution of mesoporous graphene/LiNi1/3Co1/3Mn1/3O2 composite cathode for Li-ion battery. Rare Met 40(3):521–528
Nayak PK, Erickson EM, Schipper F, Penki TR, Munichandraiah N, Adelhelm P, Sclar H, Amalraj F, Markovsky B, Aurbach D (2018) Review on challenges and recent advances in the electrochemical performance of high capacity Li- and Mn-rich cathode materials for Li-ion batteries. Adv Energy Mater 8(8):1702397
Zhao SQ, Guo ZQ, Yan K, Wan SW, He FR, Sun B, Wang GX (2021) Towards high-energy-density lithium-ion batteries: strategies for developing high-capacity lithium-rich cathode materials. Energy Storage Mater 34:716–734
Zheng JM, Gu M, Xiao J, Zuo PJ, Wang CM, Zhang JG (2013) Corrosion/fragmentation of layered composite cathode and related capacity/voltage fading during cycling process. Nano Lett13(8):3824–3830
Boulineau A, Simonin L, Colin JF, Bourbon C, Patoux S (2013) First evidence of manganese-nickel segregation and densification upon cycling in Li-rich layered oxides for lithium batteries. Nano Lett 13(8):3857–3863
Zhao TL, Gao XY, Wei ZJ, Guo KJ, Wu F, Li L, Chen RJ (2018) Three-dimensional Li1.2Ni0.2Mn0.6O2 cathode materials synthesized by a novel hydrothermal method for lithium-ion batteries. J Alloys Compd 757:16–23
Li L, Wang L, Zhang X, Xue Q, Wei L, Wu F, Chen R (2017) 3D reticular Li1.2Ni0.2Mn0.6O2 cathode material for lithium-ion batteries. ACS Appl Mater Interfaces 9(2):1516–1523
He W, Zheng H, Ju X, Li S, Ma Y, Xie Q, Wang L, Qu B, Peng D-L (2017) Multistage Li 1.2Ni0.2Mn0.6O2 micro-architecture towards high-performance cathode materials for lithium-ion batteries. Chem Electro Chem 4(12):3250–3256
Liu Y, Liu D, Zhang Z, Zheng S, Wan H, Dou A, Su M (2017) Investigation of the structural and electrochemical performance of Li1.2Ni0.2Mn0.6O2 with Cr doping. Ionics 24(8):2251–2259
Yang MC, Hu B, Geng FS, Li C, Lou XB, Hu BW (2018) Mitigating voltage decay in high-capacity Li1.2Ni0.2Mn0.6O2 cathode material by surface K+ doping. Electrochimica Acta 291:278–286
Liu H, Tao L, Wang W, Zhang B, Su M (2018) Effects of raw materials on the electrochemical performance of Na-doped Li-rich cathode materials Li[Li0.2Ni0.2Mn0.6]O2. Ionics 25(3):959–968
Li XN, Cao ZX, Yue HY, Wang QX, Zhang HS, Yang ST (2019) Tuning primary particle growth of Li1.2Ni0.2Mn0.6O2 by Nd-modification for improving the electrochemical performance of lithium ion batteries. Acs Sustain Chem Eng 7(6):5946–5952
Li M, Wang HY, Zaho LM, Zhang F, He DN (2019) The combined effect of CaF2 and graphite two-layer coatings on improving the electrochemical performance of Li-rich layer oxide material. J Solid State Chem 272:38–46
Chong SK, Chen YZ, Yan WW, Guo SW, Tan Q, Wu YF, Jiang T, Liu YN (2016) Suppressing capacity fading and voltage decay of Li-rich layered cathode material by a surface nano-protective layer of CoF2 for lithium-ion batteries. J Power Sources 332:230–239
Zhu W, Chong SK, Sun JJ, Guo SW, Tai ZG, Wang YJ, Liu YN (2019) The enhanced electrochemical performance of Li1.2Ni0.2Mn0.6O2 through coating MnF2 nano protective layer. Energy Technol 7(10):1702397
Liu YJ, Gao YY, Wang QL, Dou AC (2014) Influence of coated MnO2 content on the electrochemical performance of Li1.2Ni0.2Mn0.6O2 cathodes. Ionics 20(6):825–831
Mu KC, Cao YB, Hu GR, Du K, Yang H, Gan ZG, Peng ZD (2018) Enhanced electrochemical performance of Li-rich cathode Li1.2Ni0.2Mn0.6O2 by surface modification with WO3 for lithium ion batteries. Electrochimica Acta 273:88–97
Zhao YJ, Lv Z, Xu T, Li JX (2017) SiO2 coated Li1.2Ni0.2Mn0.6O2 as cathode materials with rate performance, HF scavenging and thermal properties for Li-ion batteries. J Alloys Compd 715:105–111
Yan PF, Zheng JM, Zhang XF, Xu R, Amine K, Xiao J, Zhang JG, Wang CM (2016) Atomic to nanoscale investigation of functionalities of an Al2O3 coating layer on a cathode for enhanced battery performance. Chem Mater 28(3):857–863
Liu YJ, Wang QL, Wang XQ, Wang TC, Gao YY, Su MR, Dou AC (2015) Improved electrochemical performance of Li1.2Ni0.2Mn0.6O2 cathode material with fast ionic conductor Li3VO4 coating. Ionics 21(10):2725–2733
Liu YJ, Wang QL, Zhang ZQ, Dou AC, Pan J, Su MR (2016) Investigation the electrochemical performance of layered cathode material Li1.2Ni0.2Mn0.6O2 coated with Li4Ti5O12. Adv Powder Technol 27(4):1481–1487
Liu YJ, Fan XJ, Zhang ZQ, Wu HH, Liu DM, Dou AC, Su MR, Zhang QB, Chu DW (2019) Enhanced electrochemical performance of Li-rich layered cathode materials by combined Cr doping and LiAlO2 coating. Acs Sustain Chem Eng 7(2):2225–2235
Zhang LL, Chen JJ, Cheng S, Xiang HF (2016) Enhanced electrochemical performances of Li1.2Ni0.2Mn0.6O2 cathode materials by coating LiAlO2 for lithium-ion batteries. Ceram Int 42(1):1870–1878
Chen DR, Zheng F, Li L, Chen M, Zhong XX, Li WS, Lu L (2017) Effect of Li3PO4 coating of layered lithium-rich oxide on electrochemical performance. J Power Sources 341:147–155
Bian XF, Fu Q, Bie XF, Yang PL, Qiu HL, Pang Q, Chen G, Du F, Wei YJ (2015) Improved electrochemical performance and thermal stability of Li-excess Li1.18Co0.15Ni0.15Mn0.52O2 cathode material by Li3PO4 surface coating. Electrochim Acta 174:875–884
Gan QM, Qin N, Wang ZY, Li ZQ, Zhu YH, Li YZ, Gu S, Yuan HM, Luo W, Lu L, Xu ZH, Lu ZG (2020) Revealing mechanism of Li3PO4 coating suppressed surface oxygen release for commercial Ni-rich layered cathodes. Acs Appl Energy Mater 3(8):7445–7455
Lee SW, Kim MS, Jeong JH, Kim DH, Chung KY, Roh KC, Kim KB (2017) Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: Improved thermal stability and high-voltage performance. J Power Sources 360:206–214
Sun Y, Zan L, Zhang Y (2019) Effects of Li3PO4 additive on the electrochemical properties of Li2FeSiO4 as cathode material for lithium-ion batteries. J Mater Sci: Mater Electron 30(16):15582–15591
Kresse G, Furthmuller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169–11186
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Kresse G, Joubert J (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758–1775
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188–5192
Wu FL, Kim GT, Diemant T, Kuenzel M, Schur AR, Gao XP, Qin BS, Alwast D, Jusys Z, Behm RJ, Geiger D, Kaiser U, Passerini S (2020) Reducing capacity and voltage decay of Co-free Li1.2Ni0.2Mn0.6O2 as positive electrode material for lithium batteries employing an ionic liquid-based electrolyte. Adv Energy Mater 10(34):2001830
Qing RP, Shi JL, Xiao DD, Zhang XD, Yin YX, Zhai YB, Gu L, Guo YG (2016) Enhancing the kinetics of Li-rich cathode materials through the pinning effects of gradient surface Na+ doping. Adv Energy Mater 6(6):1501914
Lee Y, Lee J, Lee KY, Mun J, Lee JK, Choi W (2016) Facile formation of a Li3PO4 coating layer during the synthesis of a lithium-rich layered oxide for high-capacity lithium-ion batteries. J Power Sources 315:284–293
Carroll KJ, Qian D, Fell C, Calvin S, Veith GM, Chi MF, Baggetto L, Meng YS (2013) Probing the electrode/electrolyte interface in the lithium excess layered oxide Li1.2Ni0.2Mn0.6O2. Phys Chem Chem Phys 15(26):11128–11138
Wang QY, Liu J, Murugan AV, Manthiram A (2009) High capacity double-layer surface modified LiLi0.2Mn0.54Ni0.13Co0.13O2 cathode with improved rate capability. J Mater Chem 19(28):4965–4972
Lei CH, Bareno J, Wen JG, Petrov I, Kang SH, Abraham DP (2008) Local structure and composition studies of Li1.2Ni0.2Mn0.6O2 by analytical electron microscopy. J Power Sources 178(1):422–433
Armstrong AR, Holzapfel M, Novak P, Johnson CS, Kang SH, Thackeray MM, Bruce PG (2006) Demonstrating oxygen loss and associated structural reorganization in the lithium battery cathode LiNi0.2Li0.2Mn0.6O2. J Am Chem Soc 128(26):8694–8698
Wu F, Li N, Su YF, Lu HQ, Zhang LJ, An R, Wang Z, Bao LY, Chen S (2012) Can surface modification be more effective to enhance the electrochemical performance of lithium rich materials? J Mater Chem 22(4):1489–1497
Liu JL, Hou MY, Yi J, Guo SS, Wang CX, Xia YY (2014) Improving the electrochemical performance of layered lithium-rich transition-metal oxides by controlling the structural defects. Energy Environ Sci 7(2):705–714
Shang HF, Ning FH, Li B, Zuo YX, Lu SG, Xia DG (2018) Suppressing voltage decay of a lithium-rich cathode material by surface enrichment with atomic ruthenium. ACS Appl Mater Interfaces 10(25):21349–21355
Cao J, Huang HJ, Qu YF, Tang WJ, Yang ZH, Zhang WX (2021) Construction of a hetero-epitaxial nanostructure at the interface of Li-rich cathode materials to boost their rate capability and cycling performances. Nanoscale 13(48):20488–20497
Huang, C, Wang ZJ, Fang ZQ, Zhao SX, Ci LJ (2021) Achieving high initial coulombic efficiency and low voltage dropping in Li-rich Mn-based cathode materials by metal-organic frameworks-derived coating. J Power Sources 499:229967
Bard AJ, Faulkner JR (2001) Electrochemical methods, 2nd edn. Wiley, New York, p 231
Xu K (2004) Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev 104(10):4303–4417
Funding
This study was financially supported by the National Science Foundation of China (Grant No. 52102111), Research and Innovation Initiatives of WHPU (No. 2023Y26 and No. 2023Y25), Nature Science Foundation of Hubei Province (No. 2021CFB218), the Key Research and Development Project of Hubei Province (No. 2020BBB068) and Nature Science Foundation of Hubei Province (No. 2020CFB400).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Y., Zhang, X., Cheng, J. et al. Surface modification with lithium-ion conductor Li3PO4 to enhance the electrochemical performance of lithium-rich layered Li1.2Ni0.2Mn0.6O2. Ionics 29, 2141–2152 (2023). https://doi.org/10.1007/s11581-023-04959-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04959-3