Abstract
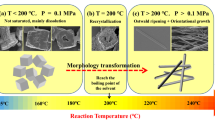
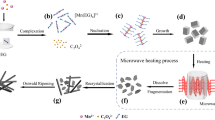
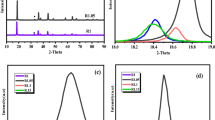
Manganese oxalate is a low-cost and high-capacity anode for lithium-ion batteries (LIBs). However, its performance is limited by the low conductivity and the volume change during charge/discharge processes. Herein, polymorphic manganese oxalates were controllably synthesized by a solvothermal process. Monoclinic α-MnC2O4·2H2O with space group C2/c can be prepared with the reactant concentration below 0.2 mol·L−1, while orthonormal MnC2O4·3H2O with space group Pcaa can be prepared with the reactant concentration above 0.2 mol·L−1. After removing crystal water, MnC2O4·2H2O and MnC2O4·3H2O are transformed into orthonormal MnC2O4. When the reactant concentration increases from 0.1 to 0.3 mol·L−1, manganese oxalate changes from rods to cubes, and its specific surface area and pore volume first increase and then decrease. Mesoporous MnC2O4 rod prepared at 0.2 mol·L−1 has a larger specific surface area and pore volume. This rod-like sample can maintain 920 and 790 mAh·g−1 after 300 cycles 2 and 5 A·g−1, respectively, exhibiting higher specific capacity, better cycle stability, and better rate performance. Therefore, the prepared mesoporous MnC2O4 rod can potentially apply in high-energy–density LIBs.










Similar content being viewed by others
References
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Larcher D, Tarascon J (2015) Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 7:19–29
Gao G, Zhang KY, Wang QW, Zhang LB, Cui DF, Yao YC (2022) Metal oxalate-based anode materials: a new choice for energy storage materials applied in metal ion batteries. Prog Chem 34(2):434–446
He QQ, Wang HY, Zhao X, Chen LY (2021) Recent advances of transition metal oxalate-based micro- and nanomaterials for electrochemical energy storage: a review. Mater Today Chem 22:100564
Wang SH, Yuan ZY, Wu WL, Li YL, Mi HW, Ren XZ, Zhang PX, Sun LN (2021) Facile synthesis of a scale-like NiO/Ni composite anode with boosted electrochemical performance for lithium-ion batteries. J Alloys Compd 862:158012
Zhang YM, Li SJ, Cheng LL, Li YL, Ren XZ, Zhang PX, Sun LN, Yang HY (2021) Confining Sb2Se3 nanorod yolk in a mesoporous carbon shell with an in-built buffer space for stable Li-ion batteries. J Mater Chem A 9(6):3388–3397
Wu XH, Guo JH, Mcdonald MJ, Li SG, Xu BB, Yang Y (2015) Synthesis and characterization of urchin-like Mn0.33Co0.67C2O4 for Li-ion batteries: role of SEI layers for enhanced electrochemical properties. Electrochim Acta 163:93–101
Yeoh JS, Armer CF, Lowe A (2018) Transition metal oxalates as energy storage materials. A review. Mater Today Energy 9:198–222
Zhang YN, Li SS, Kuai HX, Long YF, Lv XY, Su J, Wen YX (2021) Proton solvent-controllable synthesis of manganese oxalate anode material for lithium-ion batteries. RSC Adv 11:23259–23269
Lu Y, Yu L, Lou X, Wen D (2018) Nanostructured conversion-type anode materials for advanced lithium-ion batteries. Chem 4:972–996
Kang WP, Shen Q (2013) The shape-controlled synthesis and novel lithium storage mechanism of as-prepared CuC2O4·xH2O nanostructures. J Power Sources 238:203–209
Aragón MJ, León B, Pérez Vicente C, Tirado JL (2009) On the use of transition metal oxysalts as conversion electrodes in lithium-ion batteries. J Power Sources 189:823–827
Aragón MJ, León B, Pérez Vicente C, Tirado JL, Chadwick AV, Berko A, Beh S (2009) Cobalt oxalate nanoribbons as negative-electrode material for lithium-ion batteries. Chem Mater 21:1834–1840
Aragón MJ, León B, Pérez Vicente C, Tirado JL (2008) Synthesis and electrochemical reaction with lithium of mesoporous iron oxalate nanoribbons. Inorg Chem 47:10366–10371
Zhang YH, Lu ZX, Guo MQ, Bai ZC, Tang B (2016) Porous CoC2O4 nanorods as high performance anode material for lithium ion batteries. JOM 68:2952–2957
Ang WA, Cheah YL, Wong CL, Prasanth R, Hng HH, Madhavi S (2013) Mesoporous cobalt oxalate nanostructures as high-performance anode materials for lithium-ion batteries: ex situ electrochemical mechanistic study. J Phys Chem C 117:16316–16325
Xu JM, He L, Liu H, Han T, Wang YJ, Zhang CJ, Zhang YH (2015) Controlled synthesis of porous anhydrous cobalt oxalate nanorods with high reversible capacity and excellent cycling stability. Electrochim Acta 170:85–91
Zhang KY, Xu RH, Wei RH, Li Y, Wang YK, Zhang YN, Dai YN, Yao YC (2020) Tunable polymorph and morphology synthesis of iron oxalate nanoparticles as anode materials for lithium ion batteries. Mater Chem Phys 243:122676
Ang WAE, Cheah YL, Wong CL, Hng HH, Madhavi S (2015) One-pot solvothermal synthesis of Co1−xMnxC2O4 and their application as anode materials for lithium-ion batteries. J Alloy Compd 638:324–333
Donkova B, Avdeev G (2015) Synthesis and decomposition mechanism of γ-MnC2O4·2H2O rods under non-isothermal and isothermal conditions. J Therm Anal Calorim 121:567–577
Fu XC, Wang CG, Li MT (2005) Catena-poly[[[diaquamanganese(II)]-μ-oxalato] monohydrate]. Acta Crystallogr E: Struct Rep Online 61(7):m1348–m1349
Donkova B, Mehandjiev D (2004) Mechanism of decomposition of manganese(II) oxalate dihydrate and manganese(II) oxalate trihydrate. Thermochim Acta 421:141–149
Pencheva J, Donkova B, Djarova M, Maksimova S (2005) Adsorption of 3d-elements onto zinc oxalate dihydrate in aqueous oxalate solutions. Cryst Res Technol 40:370–379
Aoun-Habbache M, Guillemet-Fritsch S, Lemaître J, Jones A (2005) Investigation of nucleation and crystal growth kinetics of nickel manganese oxalates. J Cryst Growth 279:531–539
Murakoshi K, Hosokawa H, Tanaka N, Saito M, Wada Y, Yanagida S, Sakata T, Mori H (1998) Phase transition of ZnS nanocrystallites induced by surface modification at ambient temperature and pressure confirmed by electron diffraction. Chem Comm 1998:321–322
Yang Y, He L, Lu JF, Liu ZY, Wang NY, Su J, Long YF, Lv XY, Wen YX (2019) Rapid assemble of MnC2O4 microtubes using a microchannel reactor and their use as an anode material for lithium-ion batteries. Electrochim Acta 321:134673
Aragón MJ, León B, Serrano T, Pérez Vicente C, Tirado JL (2011) Synergistic effects of transition metal substitution in conversion electrodes for lithium-ion batteries. J Mater Chem 21:10102
Wu WY, Song Y, Li YZ, You XZ (2005) One-dimensional structure and long-range antiferromagnetic behaviour of manganese (II) oxalate trihydrate: {[Mn(μ-ox)(H2O)2]·H2O}n. Inorg Chem Commun 8:732–736
Zhang YN, Xue LY, Zhang Y, Su J, Long YF, Lü XY, Wen YX (2021) Synthesis and electrochemical performance of mesoporous MnC2O4 nanorod/rGO composite anode for lithium-ion batteries. J Mater Sci Mater Electron 32:15069–15079
Joo J, Chow BY, Prakash M, Boyden ES, Jacobson JM (2011) Face-selective electrostatic control of hydrothermal zinc oxide nanowire synthesis. Nat Mater 10:596–601
He Y, Yanagida T, Nagashima K, Zhuge F, Meng G, Xu B, Klamchuen A, Rahong S, Kanai M, Li X, Suzuki M, Kai S, Kawai T (2013) Crystal-plane dependence of critical concentration for nucleation on hydrothermal ZnO nanowires. J Phys Chem C 117:1197–1203
Muttakin M, Mitra S, Thu K, Ito K, Saha BB (2018) Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms. Int J Heat Mass Tran 122:795–805
López MC, Tirado JL, Pérez Vicente C (2013) Structural and comparative electrochemical study of M(II) oxalates, M = Mn, Fe Co, Ni, Cu, Zn. J Power Sources 227:65–71
He L, Xue LY, Kuai HX, Su J, Long YF, Lv XY, Wen YX (2021) N, N-dimethylacetamide–water mixed solvent synthesis of mesoporous MnC2O4 rod as high performance anode material for lithium-ion batteries. Ionics 27:1413–1422
Ang WA, Gupta N, Prasanth R, Madhavi S (2012) High-performing mesoporous iron oxalate anodes for lithium-ion batteries. ACS Appl Mater Int 4:7011–7019
Kim H, Choi W, Yoon J, Um JH, Lee W, Kim J, Cabana J, Yoon W (2020) Exploring anomalous charge storage in anode materials for next-generation Li rechargeable batteries. Chem Rev 120:6934–6976
Pu XJ, Zhao D, Fu CL, Chen ZX, Cao SN, Wang CS, Cao YL (2021) Understanding and calibration of charge storage mechanism in cyclic voltammetry curves. Angew Chem Int Edit 60:21310–21318
Fleischmann S, Mitchell JB, Wang R, Zhan C, Jiang D, Presser V, Augustyn V (2020) Pseudocapacitance: from fundamental understanding to high power energy storage materials. Chem Rev 120:6738–6782
Qi ZQ, Wu YD, Li XF, Qu Y, Yang YY, Mei DJ (2020) Microwave-assisted synthesis of CuC2O4·xH2O for anode materials in lithium-ion batteries with a high capacity. Ionics 26:33–42
Jia YM, Cheng AM, Ke W, Liu JW, Wang SQ, Zhao YF, Yang QH, Zhang JJ (2021) Hierarchical structure constructed by manganese oxalate framework with accurate iron doping for ultra-efficient lithium storage. Electrochim Acta 380:138217
Pu T, Li J, Jiang YQ, Huang B, Chen LY (2018) Size and crystallinity control of two-dimensional porous cobalt oxalate thin sheets: tuning surface structure with enhanced performance for aqueous asymmetric supercapacitors. Dalton T 47(28):9241–9249
Zhang KY, Li Y, Wang YK, Zhao JY, Chen XM, Dai YN, Yao YC (2020) Enhanced electrochemical properties of iron oxalate with more stable Li+ ions diffusion channels by controlling polymorphic structure. Chem Eng J 384:123281
Funding
The authors appreciate the financial support from the National Natural Science Foundation of China (51864005 and 51564002) and the Natural Science Foundation of Guangxi, China (2018GXNSFDA281014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Wei, Lj., Liu, ZZ. et al. Solvothermal controllable synthesis of polymorphic manganese oxalate anode for lithium-ion batteries. Ionics 28, 3603–3614 (2022). https://doi.org/10.1007/s11581-022-04653-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-022-04653-w