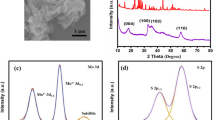
Abstract
During the past decades, lithium-sulfur batteries have obtained great attention due to their advantages as energy storage systems, which have high specific capacity and energy density. However, the real applications were hindered by many challenges, especially the shuttle effect of the polysulfide from the cathode to the anode side. This is attributed that the traditional PP films could not prevent the polysulfide migration from the cathode to the anode side. Therefore, it is an efficient method to develop a modified film, which could inhibit the polysulfide shuttle effect. Based on this idea, we prepared molybdenum sulfide–modified (MS modified) film as separators in the lithium-sulfur batteries, which were obtained by coating the molybdenum sulfide on the surface of the traditional PP films. The electrochemical results indicate that the Li–S battery by using MS–modified films exhibits excellent electrochemical performance.





Similar content being viewed by others
References
Lu Y, Gu S, Guo J, Rui K, Chen CH, Zhang SP, Jin J, Yang JH, Wen ZY (2017) Sulfonic groups originated dual-functional interlayer for high performance lithium–sulfur battery. ACS Appl Mater Interfaces 9:14878–14888
Xu N, Qian T, Liu XJ, Liu J, Chen Y, Yan CL (2017) Greatly suppressed shuttle effect for improved lithium sulfur battery performance through short chain intermediates. Nano Lett 17:538–543
Agostini M, Xiong SZ, Matic A, Hassoun J (2015) Polysulfide-containing glyme-based electrolytes for lithium sulfur battery. Chem Mater 27:4604–4611
Fan Q, Liu W, Weng Z, Sun YM, Wang HL (2015) Ternary hybrid material for high-performance lithium–sulfur battery. J Am Chem Soc 137:12946–12953
Li ML, Wahyudi W, Kumar P, Wu FY, Yang XL, Li HN, Li LJ, Ming J (2017) Scalable approach to construct free-standing and flexible carbon networks for lithium–sulfur battery. ACS Appl Mater Interfaces 9:8047–8054
He G, Evers S, Liang X, Cuisinier M, Garsuch A, Nazar LF (2013) Tailoring porosity in carbon nanospheres for lithium–sulfur battery cathodes. ACS Nano 7:10920–10930
Zhang K, Qin FR, Lai YQ, Li J, Lei XK, Wang MR, Lu H, Fang J (2016) Efficient fabrication of hierarchically porous graphene-derived aerogel and its application in lithium sulfur battery. ACS Appl Mater Interfaces 8:6072–6081
Wu F, Ye YS, Chen RJ, Qian J, Zhao T, Li L, Li WH (2015) Systematic effect for an ultralong cycle lithium–sulfur battery. Nano Lett 15:7431–7439
Du Y, Huang RK, Lin XD, Khan SK, Zheng BN, Fu RW (2020) Template-free preparation of hierarchical porous carbon nanosheets for lithium–sulfur battery. Langmuir 36:14507–14513
Li Z, Han Y, Wei JH, Wang WQ, Cao TT, Xu SM, Xu ZH (2017) Suppressing shuttle effect using Janus cation exchange membrane for high-performance lithium–sulfur battery separator. ACS Appl Mater Interfaces 9:44776–44781
Yao MJ, Wang R, Zhao ZF, Liu Y, Niu ZQ, Chen J (2018) A flexible all-in-one lithium-sulfur battery. ACS Nano 12:12503–12511
Liu J, Qian T, Wang MF, Liu XJ, Xu N, You YZ, Yan CL (2017) Molecularly imprinted polymer enables high-efficiency recognition and trapping lithium polysulfides for stable lithium sulfur battery. Nano Lett 17:5064–5070
Liu S, Li GR, Gao XP (2016) Lanthanum nitrate as electrolyte additive to stabilize the surface morphology of lithium anode for lithium–sulfur battery. ACS Appl Mater Interfaces 8:7783–7789
Mahankali K, Thangavel NK, Arava LM (2019) In situ electrochemical mapping of lithium–sulfur battery interfaces using AFM–SECM. Nano Lett 19:5229–5236
Rao DW, Yang SK, Yan XH (2020) Surface atomic configurations of MnO2 regulating the immobilization of sulfides in lithium sulfur battery. J Phys Chem C 124:5565–5573
Gueon DH, Hwang JT, Yang SB, Cho EK, Sohn K, Yang DK, Moon JH (2018) Spherical macroporous carbon nanotube particles with ultrahigh sulfur loading for lithium–sulfur battery cathodes. ACS Nano 12:226–233
Klein MJ, Veith GM, Manthiram A (2017) Rational design of lithium–sulfur battery cathodes based on experimentally determined maximum active material thickness. J Am Chem Soc 139:9229–9237
Zhang H, Qi Q, Zhang PG, Zheng W, Chen J, Zhou AG, Tian WB, Zhang W, Sun ZM (2019) Self-assembled 3D MnO2 nanosheets@delaminated-Ti3C2 aerogel as sulfur host for lithium–sulfur battery cathodes. ACS Appl Energy Mater 2:705–714
Liu J, Qian T, Wang MF, Zhou JQ, Xu N, Yan CL (2018) Use of Tween polymer to enhance the compatibility of the Li/electrolyte interface for the high-performance and high-safety quasi-solid-state lithium–sulfur battery. Nano Lett 18:4598–4605
Wang HL, Yang Y, Liang YY, Robinson JT, Li YG, Jackson A, Cui Y, Dai HJ (2011) Graphene-wrapped sulfur particles as a rechargeable lithium–sulfur battery cathode material with high capacity and cycling stability. Nano Lett 11:2644–2647
Li MY, Carter R, Douglas A, Oakes L, Pint CL (2017) Sulfur vapor-infiltrated 3D carbon nanotube foam for binder-free high areal capacity lithium–sulfur battery composite cathodes. ACS Nano 11:4877–4884
Zeng SB, Li LG, Zhao DK, Liu J, Niu WH, Wang N, Chen SW (2017) Polymer-capped sulfur copolymers as lithium–sulfur battery cathode: enhanced performance by combined contributions of physical and chemical confinements. J Phys Chem C 121:2495–2503
Huang JQ, Zhuang TZ, Zhang Q, Peng HJ, Chen CM, Wei F (2015) Permselective graphene oxide membrane for highly stable and anti-self-discharge lithium–sulfur batteries. ACS Nano 9:3002–3011
Zhang L, Wu B, Li JF (2019) Understanding interactions between lead iodide perovskite surfaces and lithium polysulfide toward new-generation integrated solar-powered lithium battery: an ab initio investigation. J Phys Chem C 123:82–90
Guo JC, Xu YH, Wang CS (2011) Sulfur-impregnated disordered carbon nanotubes cathode for lithium–sulfur batteries. Nano Lett 11:4288–4294
Zeng FL, Wang WK, Wang AB, Yuan KG, Jin ZQ, Yang YS (2015) Multidimensional polycation β-cyclodextrin polymer as an effective aqueous binder for high sulfur loading cathode in lithium–sulfur batteries. ACS Appl Mater Interfaces 7:26257–26265
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, T. Increasing the electrochemical activity performance by employing molybdenum sulfide as barrier for soluble polysulfide in Li–S batteries. Ionics 28, 1183–1187 (2022). https://doi.org/10.1007/s11581-021-04395-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04395-1