Abstract
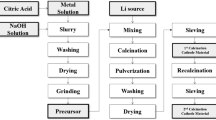
LiNi0.8Co0.15Al0.05O2 has attracted attention due to its high capacity and low cost. Herein, we report a method for the rapid and efficient preparation of NCA cathode materials using oxalic acid with freeze-drying techniques, and compare the differences of electrochemical properties for different metal concentrations. The solvent volatilization by liquid nitrogen freezing and freeze-drying techniques resulted in a more uniform mixing of metal ions while reducing the reaction time and energy consumption. The results characterized using X-ray diffraction revealed that the layered structure of the material was better and the cation mixing was lower when the metal ion concentration in the solution reached 0.15 mol L−1. The electrochemical data revealed an initial charge–discharge capacity of 195 mAh g−1 at 0.1 C, capacity retention of 88.75% for 100 cycles at 1 C, and a discharge capacity of 139.7 mAh g−1 at 5 C. In addition, the cyclic voltammetry and electrochemical impedance spectroscopy reflect the material has low electrode polarization and excellent lithium-ion diffusion coefficient. The initial material diffusion coefficient is \(2.673 \times 10^{-15}\text{ cm}^{2} \mathrm{S}^{-1}\) ; after 50 and 100 cycles, it is \(6.040 \times 10^{-16} \text{cm}^{2}\mathrm{S}^{-1}\) 10−16 cm2S−1 and \(1.704 \times 10^{-16} \text{cm}^{2}\mathrm{S}^{-1}\) respectively. The results provide a new idea for the energy-efficient synthesis of LiNi0.8Co0.15Al0.05O2.









Similar content being viewed by others
References
Zhao X, Liu B, Yang J et al (2020) Synthesizing LiNi0.5Co0.2Mn0.3O2 with microsized peanut-like structure for enhanced electrochemical properties of lithium ion batteries. J Alloy Compd 832:154464
Nie Y, Xiao W, Miao C et al (2020) Effect of calcining oxygen pressure gradient on properties of LiNi0.8Co0.15Al0.05O2 cathode materials for lithium ion batteries. Electrochim Acta 334:135654
Nitta N, Wu F, Lee JT et al (2015) Li-ion battery materials: present and future. Mater Today 18:252–264
Zhang S-S (2020) Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater 24:247–254
Sun H-H, Ryu H-H, Kim U-H et al (2020) Beyond doping and coating: prospective strategies for stable high-capacity layered Ni-rich cathodes. ACS Energy Lett 5:1136–1146
Chakraborty A, Kunnikuruvan S, Kumar S et al (2020) Layered cathode materials for lithium-ion batteries: review of computational studies on LiNi1–x–yCoxMnyO2 and LiNi1–x–yCoxAlyO2. Chem Mate 32:915–952
Ryu H-H, Park N-Y, Seo J-H et al (2020) A highly stabilized Ni-rich NCA cathode for high-energy lithium-ion batteries. Mater Today 36:73–82
Li W, Lee S, Manthiram A (2020) High-nickel NMA: a cobalt-free alternative to NMC and NCA cathodes for lithium-ion batteries. Adv Mater 32:2002718
Nie Y, Xiao W, Miao C et al (2020) Boosting the electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode materials in-situ modified with Li1.3Al0.3Ti1.7(PO4)3 fast ion conductor for lithium-ion batteries. Electrochim Acta 353:136477
Xia S, Huang W, Shen X et al (2020) Rearrangement on surface structures by boride to enhanced cycle stability for LiNi0.80Co0.15Al0.05O2 cathode in lithium ion batteries. J Energy Chem 45:110–118
Hwang S, Chang W, Kim SM et al (2014) Investigation of changes in the surface structure of LixNi0.8Co0.15Al0.05O2 cathode materials induced by the initial charge. Chem Mater 26:1084–1092
Ito S, Fujiki S, Yamada T et al (2014) A rocking chair type all-solid-state lithium ion battery adopting Li2O–ZrO2 coated LiNi0.8Co0.15Al0.05O2 and a sulfide based electrolyte. J Power Sources 248:943–950
Dong H, Li S, Liu H et al (2019) Facile synthesis and electrochemical properties of LiNi0.8Co0.15Al0.05O2 with enlarged exposed active planes for Li-ion batteries. Ionics 25:827–834
Zhang J, Xu S, Hamad KI et al (2020) High retention rate NCA cathode powders from spray drying and flame assisted spray pyrolysis using glycerol as the solvent. Powder Technol 363:1–6
Zeng T, Zhang C (2020) Influence of the total metal ions concentration and CTAB on the electrochemical properties of NCA cathode synthesized by using urea precipitant. Ionics 26:127–139
Zhang Y, Cui C, He Y et al (2021) The effect of drying methods on the structure and performance of LiNi0.5Co0.2Mn0.3O2 cathode material for lithium-ion batteries. Mater Chem Phys 262:124269
Xie H, Du K, Hu G et al (2016) The role of sodium in LiNi0.8Co0.15Al0.05O2 cathode material and its electrochemical behaviors. J Phys Chem C 120:3235–3241
Hou P, Zhang H, Deng X et al (2017) Stabilizing the electrode/electrolyte interface of LiNi0.8Co0.15Al0.05O2 through tailoring aluminum distribution in microspheres as long-life, high-rate, and safe cathode for lithium-ion batteries. Acs Appl Mater Inter 9:29643–29653
Wang Y, Jiang J, Dahn JR (2007) The reactivity of delithiated Li(Ni1/3Co1/3Mn1/3)O2, Li(Ni0.8Co0.15Al0.05)O2 or LiCoO2 with non-aqueous electrolyte. Electrochem Commun 9:2534–2540
Zhang Y, Qiu Z, Dong P et al (2018) One-step liquid-phase reaction to synthesize LiNi0.8Co0.15Al0.05O2 cathode material. J Mater Sci 53:13865–13874
Du Q-X, Tang Z-F, Ma X-H et al (2015) Improving the electrochemical properties of high-energy cathode material LiNi0.5Co0.2Mn0.3O2 by Zr doping and sintering in oxygen. Solid State Ionics 279:11–17
Huang Y, Huang Y, Hu X (2017) Enhanced electrochemical performance of LiNi0.8Co0.15Al0.05O2 by nanoscale surface modification with Co3O4. Electrochim Acta 231:294–299
Sun S, Liu T, Niu Q et al (2019) Improvement of superior cycle performance of LiNi0.8Co0.15Al0.05O2 cathode for lithium-ion batteries by multiple compound modifications. J Electroanal Chem 838:178–185
Zhu H, Li J, Chen Z et al (2014) Molten salt synthesis and electrochemical properties of LiNi1/3Co1/3Mn1/3O2 cathode materials. Synthetic Met 187:123–129
Han C-J, Yoon J-H, Cho W-I et al (2004) Electrochemical properties of LiNi0.8Co0.2-xAlxO2 prepared by a sol-gel method. J Power Sources 136:132–138
Lee D-J, Scrosati B, Sun Y-K (2011) Ni3(PO4)2-coated Li[Ni0.8Co0.15Al0.05]O2 lithium battery electrode with improved cycling performance at 55°C. J Power Sources 196:7742–7746
Huang W-J, Zheng J-Y, Liu J-J et al (2020) Boosting rate performance of LiNi0.8Co0.15Al0.05O2 cathode by simply mixing lithium iron phosphate. J Alloy Compd 827:154296
Zhou J, Wang Q, Zhang M et al (2020) In situ formed Li5AlO4-coated LiNi0.8Co0.1Mn0.1O2 cathode material assisted by hydrocarbonate with improved electrochemical performance for lithium-ion batteries. Electrochim Acta 353:136541
Zhang N, Zhang X, Shi E et al (2018) In situ X-ray diffraction and thermal analysis of LiNi0.8Co0.15Al0.05O2 synthesized via co-precipitation method. J Energy Chem 27:1655–1660
Zhang X, Liu G, Li S et al (2019) Preparation of a homogeneous Li3PO4 coating and its effect on the electrochemical properties of LiNi0.8Co0.15Al0.05O2. J Electron Mater 48:4443–4451
Xia H, Liu C, Shen L et al (2020) Structure and thermal stability of LiNi0.8Co0.15Al0.05O2 after long cycling at high temperature. J Power Sources 450:227695
Liang M, Sun Y, Song D et al (2019) Superior electrochemical performance of quasi-concentration-gradient LiNi0.8Co0.15Al0.05O2 cathode material synthesized with multi-shell precursor and new aluminum source. Electrochim Acta 300:426–436
Cao C, Zhang J, Xie X et al (2019) A novel method for the modification of LiNi0.8Co0.15Al0.05O2 with high cycle stability and low pH. J Solid State Electr 23:1351–1358
Chen T, Li X, Wang H et al (2018) The effect of gradient boracic polyanion-doping on structure, morphology, and cycling performance of Ni-rich LiNi0.8Co0.15Al0.05O2 cathode material. J Power Sources 374:1–11
Xi Z, Wang Z, Peng W et al (2020) Effect of copper and iron substitution on the structures and electrochemical properties of LiNi0.8Co0.15Al0.05O2 cathode materials. Energy Sci Eng 8:1868–1879
Purwanto A, Yudha CS, Ikhwan Muhammad K et al (2020) Synthesis of LiNi0.8Co0.15Al0.05O2 cathode material via flame-assisted spray pyrolysis method. Adv Powder Technol 31:1674–1681
Liao K, Wei H, Shi P et al (2020) An exquisite electrode material using aramid nanofibers with enhanced discharge capacity and catalytic conversion of polysulfides. J Mater Chem A 8(40):21163–21172
Du J, Gao S, Shi P et al (2020) Three-dimensional carbonaceous for potassium ion batteries anode to boost rate and cycle life performance. J Power Sources 451:227727
Dai R, Zhang Y, Fan J et al (2020) Enhanced electrochemical kinetics and polysulfide traps of bifunctional perovskite promoter for highly stable lithium-sulfur batteries. ACS Sustain Chem Eng 8(50):18636–18645
He H, Chai Y, Zhang X et al (2021) A 2D–3D co-conduction effect in PEO-based all-solid-state batteries for long term cycle stability. J Mater Chem A 9(14):9214–9227
Zhang Y, Gu R, Zheng S et al (2019) Long-life Li–S batteries based on enabling the immobilization and catalytic conversion of polysulfides. J Mater Chem A 7(38):21747–21758
Zheng S, Zhang H, Fan J et al (2020) Improving electrochemical performance and safety of lithium-sulfur batteries by a “bulletproof vest.” ACS Appl Mater Inter 12(46):51904–51916
Huang Y, Cao S, Xie X et al (2020) Improving the structure and cycling stability of Ni-rich layered cathodes by dual modification of yttrium doping and surface coating. ACS Appl Mater Inter 12(17):19483–19494
Jamil S, Wang G, Yang L et al (2020) Suppressing H2–H3 phase transition in high Ni–low Co layered oxide cathode material by dual modification. J Mater Chem A 8(40):21306–21316
Jamil S, Ran Q, Yang L, et al (2021) Improved high-voltage performance of LiNi0.87Co0.1Al0.03O2 by Li+-conductor coating. Chem Eng J 407:126442
Funding
This work was financially supported by the National Natural Science Foundation of China (NSFC,51606102) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, Z., Cao, X., Cui, C. et al. Improved preparation efficiency and electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material by oxalic acid and freeze-drying. Ionics 27, 4663–4672 (2021). https://doi.org/10.1007/s11581-021-04228-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04228-1