Abstract
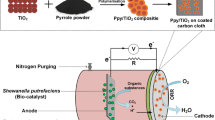
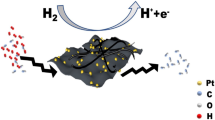
Landfill leachate is a type of complex organic wastewater, which is prone to cause serious negative impacts on the ecological environment and human health. Using fuel cells to remove the organic matter in landfill leachate is an efficient and feasible approach. The fuel cells are primarily limited by the cathodic oxygen reduction reaction (ORR) with sluggish kinetics, which requires catalysts with high electrochemical properties to improve ORR activity. Herein, Pt-Te nanowires (NWs) with high activity and durability were synthesized. The transmission electron microscope (TEM) images indicated that Pt nanoparticles were uniformly decorated in the Te nanowires to form active sites. The electrochemical activities of the catalysts were investigated using the rotating disk electrode (RDE) system. Compared with Pt/C and pure Te NWs catalysts, the Pt-Te NWs exhibited a stronger reduction peak and higher current density for ORR. Additionally, the fuel cell coupled with Pt-Te NWs possessed a high performance on the removal of the organic matter.








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current investigation are available from the corresponding author on reasonable request.
References
Zhong H, Tian Y, Yang Q, Brusseau ML, Yang L, Zeng G (2017) Degradation of landfill leachate compounds by persulfate for groundwater remediation. Chem Eng J 307:399–407. https://doi.org/10.1016/j.cej.2016.08.069
Hassan M, Zhao Y, Xie B (2016) Employing TiO 2 photocatalysis to deal with landfill leachate: current status and development. Chem Eng J 285:264–275. https://doi.org/10.1016/j.cej.2015.09.093
Bolyard SC, Reinhart DR (2016) Application of landfill treatment approaches for stabilization of municipal solid waste. Waste Manag 55:22–30. https://doi.org/10.1016/j.wasman.2016.01.024
Damiano L, Jambeck JR, Ringelberg DB (2014) Municipal solid waste landfill leachate treatment and electricity production using microbial fuel cells. Appl Biochem Biotechnol 173(2):472–485. https://doi.org/10.1007/s12010-014-0854-x
Ganesh K, Jambeck JR (2013) Treatment of landfill leachate using microbial fuel cells: alternative anodes and semi-continuous operation. Bioresour Technol 139:383–387. https://doi.org/10.1016/j.biortech.2013.04.013
Elmaadawy K, Liu B, Hu J, Hou H, Yang J (2020) Performance evaluation of microbial fuel cell for landfill leachate treatment: research updates and synergistic effects of hybrid systems. J Environ Sci (China) 96:1–20. https://doi.org/10.1016/j.jes.2020.05.005
Miao L, Yang G, Tao T, Peng Y (2019) Recent advances in nitrogen removal from landfill leachate using biological treatments - a review. J Environ Manage 235:178–185. https://doi.org/10.1016/j.jenvman.2019.01.057
Luo H, Zeng Y, Cheng Y, He D, Pan X (2020) Recent advances in municipal landfill leachate: a review focusing on its characteristics, treatment, and toxicity assessment. Sci Total Environ 703:135468. https://doi.org/10.1016/j.scitotenv.2019.135468
Deng Y, Zhu X, Chen N, Feng C, Wang H, Kuang P, Hu W (2020) Review on electrochemical system for landfill leachate treatment: performance, mechanism, application, shortcoming, and improvement scheme. Sci Total Environ 745:140768. https://doi.org/10.1016/j.scitotenv.2020.140768
Naveen BP, Mahapatra DM, Sitharam TG, Sivapullaiah PV, Ramachandra TV (2017) Physico-chemical and biological characterization of urban municipal landfill leachate. Environ Pollut 220(Pt A):1–12. https://doi.org/10.1016/j.envpol.2016.09.002
Foo KY, Hameed BH (2009) An overview of landfill leachate treatment via activated carbon adsorption process. J Hazard Mater 171(1–3):54–60. https://doi.org/10.1016/j.jhazmat.2009.06.038
Long Y, Xu J, Shen D, Du Y, Feng H (2017) Effective removal of contaminants in landfill leachate membrane concentrates by coagulation. Chemosphere 167:512–519. https://doi.org/10.1016/j.chemosphere.2016.10.016
Chys M, Oloibiri VA, Audenaert WTM, Demeestere K, Van Hulle SWH (2015) Ozonation of biologically treated landfill leachate: efficiency and insights in organic conversions. Chem Eng J 277:104–111. https://doi.org/10.1016/j.cej.2015.04.099
El-Gohary FA, Kamel G (2016) Characterization and biological treatment of pre-treated landfill leachate. Ecol Eng 94:268–274. https://doi.org/10.1016/j.ecoleng.2016.05.074
Zularisam AW, Ismail AF, Salim R (2006) Behaviours of natural organic matter in membrane filtration for surface water treatment — a review. Desalination 194(1–3):211–231. https://doi.org/10.1016/j.desal.2005.10.030
Liu M, Lü Z, Chen Z, Yu S, Gao C (2011) Comparison of reverse osmosis and nanofiltration membranes in the treatment of biologically treated textile effluent for water reuse. Desalination 281:372–378. https://doi.org/10.1016/j.desal.2011.08.023
Shah TM, Ramaswami S, Behrendt J, Otterpohl R (2017) Simultaneous removal of organics and ammonium-nitrogen from reverse osmosis concentrate of mature landfill leachate. Journal of Water Process Engineering 19:126–132. https://doi.org/10.1016/j.jwpe.2017.07.024
Calabro PS, Gentili E, Meoni C, Orsi S, Komilis D (2018) Effect of the recirculation of a reverse osmosis concentrate on leachate generation: a case study in an Italian landfill. Waste Manag 76:643–651. https://doi.org/10.1016/j.wasman.2018.03.007
Ye Z, Zhang H, Yang L, Wu L, Qian Y, Geng J, Chen M (2016) Effect of a solar Fered-Fenton system using a recirculation reactor on biologically treated landfill leachate. J Hazard Mater 319:51–60. https://doi.org/10.1016/j.jhazmat.2016.01.027
Deng Y, Chen N, Feng C, Chen F, Wang H, Feng Z, Zheng Y, Kuang P, Hu W (2019) Research on complexation ability, aromaticity, mobility and cytotoxicity of humic-like substances during degradation process by electrochemical oxidation. Environ Pollut 251:811–820. https://doi.org/10.1016/j.envpol.2019.05.047
Badwal SP, Giddey SS, Munnings C, Bhatt AI, Hollenkamp AF (2014) Emerging electrochemical energy conversion and storage technologies. Front Chem 2:79. https://doi.org/10.3389/fchem.2014.00079
Yang L, Shui J, Du L, Shao Y, Liu J, Dai L, Hu Z (2019) Carbon-based metal-free ORR electrocatalysts for fuel cells: past, present, and future. Adv Mater 31(13):e1804799. https://doi.org/10.1002/adma.201804799
Ren X, Lv Q, Liu L, Liu B, Wang Y, Liu A, Wu G (2020) Current progress of Pt and Pt-based electrocatalysts used for fuel cells. Sustainable Energy Fuels 4(1):15–30. https://doi.org/10.1039/c9se00460b
Li D, Wang C, Strmcnik DS, Tripkovic DV, Sun X, Kang Y, Chi M, Snyder JD, van der Vliet D, Tsai Y, Stamenkovic VR, Sun S, Markovic NM (2014) Functional links between Pt single crystal morphology and nanoparticles with different size and shape: the oxygen reduction reaction case. Energy Environ Sci 7(12):4061–4069. https://doi.org/10.1039/c4ee01564a
Andreadis G, Tsiakaras P (2006) Ethanol crossover and direct ethanol PEM fuel cell performance modeling and experimental validation. Chem Eng Sci 61(22):7497–7508. https://doi.org/10.1016/j.ces.2006.08.028
Hao Y, Wang X, Shen J, Yuan J, Wang AJ, Niu L, Huang S (2016) One-pot synthesis of single-crystal Pt nanoplates uniformly deposited on reduced graphene oxide, and their high activity and stability on the electrocalalytic oxidation of methanol. Nanotechnology 27(14):145602. https://doi.org/10.1088/0957-4484/27/14/145602
Liu J, Zhao S, Li C, Yang M, Yang Y, Liu Y, Lifshitz Y, Lee S-T, Kang Z (2016) Carbon nanodot surface modifications initiate highly efficient, stable catalysts for both oxygen evolution and reduction reactions. Advanced Energy Materials 6 (9). https://doi.org/10.1002/aenm.201502039
Wu Z, Lv Y, Xia Y, Webley PA, Zhao D (2012) Ordered mesoporous platinum@graphitic carbon embedded nanophase as a highly active, stable, and methanol-tolerant oxygen reduction electrocatalyst. J Am Chem Soc 134(4):2236–2245. https://doi.org/10.1021/ja209753w
Zhu Z, Bukowski B, Deskins NA, Zhou HS (2015) Bamboo shaped carbon nanotube supported platinum electrocatalyst synthesized by high power ultrasonic-assisted impregnation method for methanol electrooxidation and related density functional theory calculations. Int J Hydrogen Energy 40(5):2216–2224. https://doi.org/10.1016/j.ijhydene.2014.12.039
Cheon JY, Kim K, Sa YJ, Sahgong SH, Hong Y, Woo J, Yim S-D, Jeong HY, Kim Y, Joo SH (2016) Graphitic nanoshell/mesoporous carbon nanohybrids as highly efficient and stable bifunctional oxygen electrocatalysts for rechargeable aqueous Na-air batteries. Advanced Energy Materials 6 (7). https://doi.org/10.1002/aenm.201501794
Ma L, Sui S, Zhai Y (2009) Investigations on high performance proton exchange membrane water electrolyzer. Int J Hydrogen Energy 34(2):678–684. https://doi.org/10.1016/j.ijhydene.2008.11.022
Li H-H, Cui C-H, Zhao S, Yao H-B, Gao M-R, Fan F-J, Yu S-H (2012) Mixed-PtPd-shell PtPdCu nanoparticle nanotubes templated from copper nanowires as efficient and highly durable electrocatalysts. Adv Energy Mater 2(10):1182–1187. https://doi.org/10.1002/aenm.201200207
He DS, He D, Wang J, Lin Y, Yin P, Hong X, Wu Y, Li Y (2016) Ultrathin icosahedral Pt-enriched nanocage with excellent oxygen reduction reaction activity. J Am Chem Soc 138(5):1494–1497. https://doi.org/10.1021/jacs.5b12530
Wang XX, Swihart MT, Wu G (2019) Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat Catal 2(7):578–589. https://doi.org/10.1038/s41929-019-0304-9
Du XX, He Y, Wang XX, Wang JN (2016) Fine-grained and fully ordered intermetallic PtFe catalysts with largely enhanced catalytic activity and durability. Energy Environ Sci 9(8):2623–2632. https://doi.org/10.1039/c6ee01204c
Luo M, Zhao Z, Zhang Y, Sun Y, Xing Y, Lv F, Yang Y, Zhang X, Hwang S, Qin Y, Ma JY, Lin F, Su D, Lu G, Guo S (2019) PdMo bimetallene for oxygen reduction catalysis. Nature 574(7776):81–85. https://doi.org/10.1038/s41586-019-1603-7
Deng D, Novoselov KS, Fu Q, Zheng N, Tian Z, Bao X (2016) Catalysis with two-dimensional materials and their heterostructures. Nat Nanotechnol 11(3):218–230. https://doi.org/10.1038/nnano.2015.340
Cargnello M, Delgado Jaen JJ, Hernandez Garrido JC, Bakhmutsky K, Montini T, Calvino Gamez JJ, Gorte RJ, Fornasiero P (2012) Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 337(6095):713–717. https://doi.org/10.1126/science.1222887
Zhang Z-c, Xu B, Wang X (2014) Engineering nanointerfaces for nanocatalysis. Chem Soc Rev 43(22):7870–7886. https://doi.org/10.1039/C3CS60389J
Hoque MA, Hassan FM, Jauhar AM, Jiang G, Pritzker M, Choi J-Y, Knights S, Ye S, Chen Z (2017) Web-like 3D architecture of Pt nanowires and sulfur-doped carbon nanotube with superior electrocatalytic performance. ACS Sustainable Chemistry & Engineering 6(1):93–98. https://doi.org/10.1021/acssuschemeng.7b03580
Ding LX, Wang AL, Li GR, Liu ZQ, Zhao WX, Su CY, Tong YX (2012) Porous Pt-Ni-P composite nanotube arrays: highly electroactive and durable catalysts for methanol electrooxidation. J Am Chem Soc 134(13):5730–5733. https://doi.org/10.1021/ja212206m
Venarusso LB, Boone CV, Bettini J, Maia G (2018) Carbon-supported metal nanodendrites as efficient, stable catalysts for the oxygen reduction reaction. Journal of Materials Chemistry A 6(4):1714–1726. https://doi.org/10.1039/c7ta08964c
Mi S, Cheng N, Jiang H, Li C, Jiang H (2018) Porous Pt3Ni with enhanced activity and durability towards oxygen reduction reaction. RSC Adv 8(28):15344–15351. https://doi.org/10.1039/c8ra02219d
Grozovski V, Kasuk H, Nerut J, Härk E, Jäger R, Tallo I, Lust E (2015) Oxygen reduction at shape-controlled platinum nanoparticles and composite catalysts based on (100)Pt nanocubes on microporous-mesoporous carbon supports. ChemElectroChem 2(6):847–851. https://doi.org/10.1002/celc.201500021
Yao Z, Yue R, Zhai C, Jiang F, Wang H, Du Y, Wang C, Yang P (2013) Electrochemical layer-by-layer fabrication of a novel three-dimensional Pt/graphene/carbon fiber electrode and its improved catalytic performance for methanol electrooxidation in alkaline medium. Int J Hydrogen Energy 38(15):6368–6376. https://doi.org/10.1016/j.ijhydene.2013.02.140
Pei J, Mao J, Liang X, Zhuang Z, Chen C, Peng Q, Wang D, Li Y (2017) Ultrathin Pt–Zn nanowires: high-performance catalysts for electrooxidation of methanol and formic acid. ACS Sustainable Chemistry & Engineering 6(1):77–81. https://doi.org/10.1021/acssuschemeng.7b03234
Lv H, Wang J, Yan Z, Li B, Yang D, Zhang C (2017) Carbon-supported Pt-Co nanowires as a novel cathode catalyst for proton exchange membrane fuel cells. Fuel Cells 17(5):635–642. https://doi.org/10.1002/fuce.201700136
Zhu JP, Yu SH, He ZB, Jiang J, Chen K, Zhou XY (2005) Complex PbTe hopper (skeletal) crystals with high hierarchy. Chem Commun (Camb) 46:5802–5804. https://doi.org/10.1039/b510930b
Mayers B, Xia Y (2002) One-dimensional nanostructures of trigonal tellurium with various morphologies can be synthesized using a solution-phase approach. J Mater Chem 12(6):1875–1881. https://doi.org/10.1039/b201058e
Liang H-W, Liu S, Gong J-Y, Wang S-B, Wang L, Yu S-H (2009) Ultrathin Te nanowires: an excellent platform for controlled synthesis of ultrathin platinum and palladium nanowires/nanotubes with very high aspect ratio. Adv Mater 21(18):1850–1854. https://doi.org/10.1002/adma.200802286
Guo S, Dong S, Wang E (2010) Novel Te/Pt hybrid nanowire with nanoporous surface: a catalytically active nanoelectrocatalyst. The Journal of Physical Chemistry C 114(11):4797–4802. https://doi.org/10.1021/jp909623x
Liu JW, Xu J, Liang HW, Wang K, Yu SH (2012) Macroscale ordered ultrathin telluride nanowire films, and tellurium/telluride hetero-nanowire films. Angew Chem Int Ed Engl 51(30):7420–7425. https://doi.org/10.1002/anie.201201608
Xu W, Song J, Sun L, Yang J, Hu W, Ji Z, Yu SH (2008) Structural, electrical, and photoconductive properties of individual single-crystalline tellurium nanotubes synthesized by a chemical route: doping effects on electrical structure. Small 4(7):888–893. https://doi.org/10.1002/smll.200701227
Mukerjee S, Srinivasan S, Soriaga MP, McBreen J (1995) Role of structural and electronic properties of Pt and Pt alloys on electrocatalysis of oxygen reduction: an in situ XANES and EXAFS investigation. J Electrochem Soc 142(5):1409–1422. https://doi.org/10.1149/1.2048590
Xu C, Li Q, Liu Y, Wang J, Geng H (2012) Hierarchical nanoporous PtFe alloy with multimodal size distributions and its catalytic performance toward methanol electrooxidation. Langmuir 28(3):1886–1892. https://doi.org/10.1021/la203835n
Liu M, Zhao Z, Duan X, Huang Y (2019) Nanoscale structure design for high-performance Pt-based ORR catalysts. Adv Mater 31(6):e1802234. https://doi.org/10.1002/adma.201802234
Mayrhofer KJJ, Strmcnik D, Blizanac BB, Stamenkovic V, Arenz M, Markovic NM (2008) Measurement of oxygen reduction activities via the rotating disc electrode method: from Pt model surfaces to carbon-supported high surface area catalysts. Electrochim Acta 53(7):3181–3188. https://doi.org/10.1016/j.electacta.2007.11.057
Shinozaki K, Zack JW, Pylypenko S, Richards RM, Pivovar BS, Kocha SS (2015) Benchmarking the oxygen reduction reaction activity of Pt-based catalysts using standardized rotating disk electrode methods. Int J Hydrogen Energy 40(46):16820–16830. https://doi.org/10.1016/j.ijhydene.2015.08.024
Ding N, Chen S-F, Geng D-S, Chien S-W, An T, Hor TSA, Liu Z-L, Yu S-H, Zong Y (2015) Tellurium@ ordered macroporous carbon composite and free-standing tellurium nanowire mat as cathode materials for rechargeable lithium-tellurium batteries. Advanced Energy Materials 5 (8). https://doi.org/10.1002/aenm.201401999
He Z, Hassan M, Ju H-X, Wang R, Wang J-L, Chen J-F, Zhu J-F, Liu J-W, Yu S-H (2018) Stability and protection of nanowire devices in air. Nano Res 11(6):3353–3361. https://doi.org/10.1007/s12274-017-1932-5
Chen W, Westerhoff P, Leenheer JA, Booksh K (2003) Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ Sci Technol 37(24):5701–5710. https://doi.org/10.1021/es034354c
Hu J, Fu W, Ni F, Zhang X, Yang C, Sang J (2020) An integrated process for the advanced treatment of hypersaline petrochemical wastewater: a pilot study. Water Res 182:116019. https://doi.org/10.1016/j.watres.2020.116019
Funding
This work was financial supported by the National Natural Science Foundation of China (No. 51978239).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Xu, H., Yao, H. et al. Performance of a fuel cell coupled with Pt-Te nanowires for the removal of the organic matter in landfill leachate. Ionics 27, 5241–5250 (2021). https://doi.org/10.1007/s11581-021-04219-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04219-2