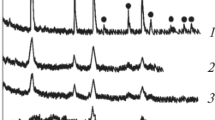
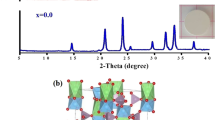
Abstract
Lithium aluminum titanium phosphate (LATP) with a formula of Li1.3Al0.3Ti1.7(PO4)3 has been regarded as one of the most promising inorganic solid-state electrolytes in all-solid-state lithium-ion batteries, and presently, to optimize the structural and electrochemical properties of its ceramic pellets is of crucial importance for potential application purposes. In this paper, chemical lithium phosphate Li3PO4 with a melting point of 837 °C is used as an agglutinant in the ceramics of as-prepared LATP crystallites, aiming to assay its functions in possible performance enhancement. At the sintering temperature of 900 °C, the self-fluxing agglutinant (10.0 wt%) in Li3PO4-LATP composite pellet is expected to fill gaps among close packed particles, and this actually induces an apparent shrinkage in the pellet diameter/thickness, a great decrease in grain-boundary resistance and an obvious increase in total Li+-ion conductivity (e.g., LATP-900 ~ 3.0 × 10−4 S cm−1; Li3PO4-LATP-900 ~ 1.2 × 10−3 S cm−1; at 60 oC). Especially, a polymorphic change of the agglutinant occurring during pellet-sintering process and the in situ formation of ion-conducting interphases may cooperatively explain the improvement of ionic conductivity.






Similar content being viewed by others
References
Bachman JC, Muy S, Grimaud A, Chang HH, Pour N, Lux SF, Paschos O, Maglia F, Lupart S, Lamp P, Giordano L, Shao-Horn Y (2016) Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem Rev 116(1):140–162. https://doi.org/10.1021/acs.chemrev.5b00563
Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J (2017) Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33:363–386. https://doi.org/10.1016/j.nanoen.2017.01.028
Luo W, Gong Y, Zhu Y, Li Y, Yao Y, Zhang Y, Fu KK, Pastel G, Lin CF, Mo Y, Wachsman ED, Hu L (2017) Reducing interfacial resistance between garnet-structured solid-state electrolyte and Li-metal anode by a germanium layer. Adv Mater 29(22):1606042. https://doi.org/10.1002/adma.201606042
Bednorz BG, Miiller KA (1988) Perovskite-type oxides-the new approach to high-Tc, superconductivity. Adv Mater 100:757–770. https://doi.org/10.1002/anie.198807351
Kotobuki M, Koishi M (2013) Preparation of Li1.5Al0.5Ti1.5(PO4)3 solid electrolyte via a sol-gel route using various Al sources. Ceram Int 39(4):4645–4649. https://doi.org/10.1016/j.ceramint.2012.10.206
Bucharsky EC, Schell KG, Hupfer T, Hoffmann MJ, Rohde M, Seifert HJ (2016) Thermal properties and ionic conductivity of Li1.3Ti1.7Al0.3(PO4)3 solid electrolytes sintered by field-assisted sintering. Ionics 22(7):1043–1049. https://doi.org/10.1007/s11581-015-1628-3
Wang Y, Liu Z, Zhu X, Tang Y, Huang F (2013) Highly lithium-ion conductive thio-LISICON thin film processed by low-temperature solution method. J Power Sources 224:225–229. https://doi.org/10.1016/j.jpowsour.2012.09.097
Choi S, Yoon I, Nichols WT, Shin D (2018) Carbon-coated Li2S cathode for improving the electrochemical properties of an all-solid-state lithium-sulfur battery using Li2S-P2S5 solid electrolyte. Ceram Int 44(7):7450–7453. https://doi.org/10.1016/j.ceramint.2018.01.104
Liang L, Sun X, Zhang J, Sun J, Hou L, Liu Y, Yuan C (2019) Sur-/interfacial regulation in all-solid-state rechargeable Li-ion batteries based on inorganic solid-state electrolytes: advances and perspectives. Mater Horiz 6:871–910. https://doi.org/10.1039/c8mh01593g
Monchak M, Hupfer T, Senyshyn A, Boysen H, Chernyshov D, Hansen T, Schell KG, Bucharsky EC, Hoffmann MJ, Ehrenberg H (2016) Lithium diffusion pathway in Li1.3Al0.3Ti1.7(PO4)3 (LATP) superionic conductor. Inorg Chem 55(6):2941–2945. https://doi.org/10.1021/acs.inorgchem.5b02821
Li S, Lin Z (1983) Phase relationship and ionic conductivity of Li1+xTi2-xInxP3O12 system. Solid State Ionics 9 & 10:835-837. https://doi.org/10.1016/0167-2738(83)90098-X
Liu Y, Sun Q, Zhao Y, Wang B, Kaghazchi P, Adair KR, Li R, Zhang C, Liu J, Kuo LY, Hu Y, Sham TK, Zhang L, Yang R, Lu S, Song X, Sun X (2018) Stabilizing the interface of NASICON solid electrolyte against Li metal with atomic layer deposition. ACS Appl Mater Interfaces 10(37):31240–31248. https://doi.org/10.1021/acsami.8b06366
Kotobuki M, Koishi M, Kato Y (2013) Preparation of Li1.5Al0.5Ti1.5(PO4)3 solid electrolyte via a co-precipitation method. Ionics 19:1945–1948. https://doi.org/10.1007/s11581-013-1000-4
Arbi K, Hoelzel M, Kuhn A, Garcia-Alvarado F, Sanz J (2013) Structural factors that enhance lithium mobility in fast-ion Li1+xTi2–xAlx (PO4)3 (0 ≤ x ≤ 0.4) conductors investigated by neutron diffraction in the temperature range 100-500 K. Inorg Chem 52(16):9290–9296. https://doi.org/10.1021/ic400577v
Aono H, Sugimoto E (1990) Ionic conductivity of solid electrolytes based on lithium titanium phosphate. J Electrochem Soc 137:1023–1027. https://doi.org/10.1149/1.2086597
Aono H, Sugimoto E (1993) The electrical properties of ceramic electrolytes for LiMxTi2-x (PO4)3 + yLi2O, M = Ge, Sn, Hf, and Zr systems. J Electrochem Soc 140:1827–1833. https://doi.org/10.1149/1.2220723
Li Y, Wang Z, Li C, Cao Y, Guo X (2014) Densification and ionic-conduction improvement of lithium garnet solid electrolytes by flowing oxygen sintering. J Power Sources 248:642–646. https://doi.org/10.1016/j.jpowsour.2013.09.140
El-Shinawi H, Paterson GW, MacLaren DA, Cussen EJ, Corr SA (2017) Low-temperature densification of Al-doped Li7La3Zr2O12: a reliable and controllable synthesis of fast-ion conducting garnets. J Mater Chem A 5(1):319–329. https://doi.org/10.1039/c6ta06961d
Li Y, Chen X, Dolocan A, Cui Z, Xin S, Xue L, Xu H, Park K, Goodenough JB (2018) Garnet electrolyte with an ultralow interfacial resistance for Li-metal batteries. J Am Chem Soc 140(20):6448–6455. https://doi.org/10.1021/jacs.8b03106
Kwatek K, Ślubowska W, Trébosc J, Lafon O, Nowiński JL (2020) Structural and electrical properties of ceramic Li-ion conductors based on Li1.3Al0.3Ti1.7(PO4)3-LiF. J Eur Ceram Soc 40(1):85–93. https://doi.org/10.1016/j.jeurceramsoc.2019.08.032
Lu Y, Yang F, Wang GGX, Shi S, Liu Y (2020) Enhanced electrochemical properties and thermal stability of Zr4+ doped Li1.20Mn0.54Ni0.13Co0.13O2 cathode material by a Li ion conductor of Li3PO4 surface coating. Appl Sur Sci 521:146338. https://doi.org/10.1016/j.apsusc.2020.146338
Z-b X, Chen S, Guo M-m (2011) Influence of Li3PO4 addition on properties of lithium ion-conductive electrolyte Li1.3Al0.3Ti1.7(PO4)3. Trans Nonferrous Metals Soc China 21(11):2454–2458. https://doi.org/10.1016/s1003-6326(11)61036-4
Lee SD, Jung KN, Kim H, Shin HS, Song SW, Park MS, Lee JW (2017) Composite electrolyte for all-solid-state lithium batteries: low-temperature fabrication and conductivity enhancement. ChemSusChem 10(10):2175–2181. https://doi.org/10.1002/cssc.201700104
Xie H, Li C, Kan WH, Avdeev M, Zhu C, Zhao Z, Chu X, Mu D, Wu F (2019) Consolidating the grain boundary of the garnet electrolyte LLZTO with Li3BO3 for high-performance LiNi0.8Co0.1Mn0.1O2/LiFePO4 hybrid solid batteries. J Mater Chem A 7(36):20633–20639. https://doi.org/10.1039/c9ta03263k
Zhao G, Suzuki K, Seki T, Sun X, Hirayama M, Kanno R (2020) High lithium ionic conductivity of γ-Li3PO4-type solid electrolytes in Li4GeO4-Li4SiO4-Li3VO4 quasi-ternary system. J Solid State Chem 292:121651. https://doi.org/10.1016/j.jssc.2020.121651
Deng T, Ji X, Zhao Y, Cao L, Li S, Hwang S, Luo C, Wang P, Jia H, Fan X, Lu X, Su D, Sun X, Wang C, Zhang JG (2020) Tuning the anode-electrolyte interface chemistry for garnet-based solid-state Li metal batteries. Adv Mater 32(23):2000030. https://doi.org/10.1002/adma.202000030
Zemann VJ (1960) Die kristallstruktur von lithiumphosphat, Li3PO4. Acta Cryst 13:863–867. https://doi.org/10.1107/S0365110X60002132
Li C, Wang J, Chang Z, Yin Y, Yang X, Zhang X (2018) Preparation and characterization of PAN-LATP composite solid-state electrolyte. Sci Sin Chim 48(8):964–971. https://doi.org/10.1360/n032018-00062
Su M, Wang Z, Li X, Guo H, Peng W (2013) Preparation and characterization of lithium fast ion conductor Li1.3Al0.3Ti1.7(PO4)3 ultrafine powders by sol-gel method. Trans Nonferrous Metals Soc China 2:469–473
Bucharsky EC, Schell KG, Hintennach A, Hoffmann MJ (2015) Preparation and characterization of sol-gel derived high lithium ion conductive NZP-type ceramics Li1+xAlxTi2−x (PO4)3. Solid State Ionics 274:77–82. https://doi.org/10.1016/j.ssi.2015.03.009
Luo J-Y, Chen L-J, Zhao Y-J, He P, Xia Y-Y (2009) The effect of oxygen vacancies on the structure and electrochemistry of LiTi2(PO4)3 for lithium-ion batteries: a combined experimental and theoretical study. J Power Sources 194(2):1075–1080. https://doi.org/10.1016/j.jpowsour.2009.06.050
Aatiq A, Ménétrier M, Croguennec L, Suard E, Delmas C (2002) On the structure of Li3Ti2(PO4)3. J Mater Chem 12(10):2971–2978. https://doi.org/10.1039/b203652p
Soman S, Iwai Y, Kawamura J, Kulkarni A (2011) Crystalline phase content and ionic conductivity correlation in LATP glass-ceramic. J Solid State Electrochem 16(5):1761–1766. https://doi.org/10.1007/s10008-011-1592-4
Aravindan V, Chuiling W, Reddy MV, Rao GV, Chowdari BV, Madhavi S (2012) Carbon coated nano-LiTi2(PO4)3 electrodes for non-aqueous hybrid supercapacitors. Phys Chem Chem Phys 14(16):5808–5814. https://doi.org/10.1039/c2cp40603a
Key B, Schroeder DJ, Ingram BJ, Vaughey JT (2012) Solution-based synthesis and characterization of lithium-ion conducting phosphate ceramics for lithium metal batteries. Chem Mater 24(2):287–293. https://doi.org/10.1021/cm202773d
Lin J, Wu L, Huang Z, Xu X, Liu J (2020) La2Zr2O7 and MgO co-doped composite Li-garnet solid electrolyte. J Energy Chem 40:132–136. https://doi.org/10.1016/j.jechem.2019.02.003
Zhao E, Ma F, Jin Y, Kanamura K (2016) Pechini synthesis of high ionic conductivity Li1.3Al0.3Ti1.7(PO4)3 solid electrolytes: the effect of dispersant. J Alloys Compd 680:646–653. https://doi.org/10.1016/j.jallcom.2016.04.173
Duluard S, Paillassa A, Lenormand P, Taberna P-L, Simon P, Rozier P, Ansart F, Ihlefeld J (2017) Dense on porous solid LATP electrolyte system: preparation and conductivity measurement. J Am Chem Soc 100(1):141–149. https://doi.org/10.1111/jace.14451
Arbi K, Mandal S, Rojo JM, Sanz J (2002) Dependence of ionic conductivity on composition of fast ionic conductors Li1+xTi2-xAlx (PO4)3, (0 ≤ x ≤ 0.7). A parallel NMR and electric impedance study. Chem Mater 14:1091–1097. https://doi.org/10.1021/cm010528i
Šalkus T, Barre M, Kežionis A, Kazakevičius E, Bohnke O, Selskienė A, Orliukas AF (2012) Ionic conductivity of Li1.3Al0.3-xScxTi1.7(PO4)3 (x = 0, 0.1, 0.15, 0.2, 0.3) solid electrolytes prepared by Pechini process. Solid State Ionics 225:615–619. https://doi.org/10.1016/j.ssi.2012.03.045
Waetzig K, Rost A, Langklotz U, Matthey B, Schilm J (2016) A An explanation of the microcrack formation in Li1.3Al0.3Ti1.7(PO4)3 ceramics. J Eur Ceram Soc 36(8):1995–2001. https://doi.org/10.1016/j.jeurceramsoc.2016.02.042
Liu Y, Liu J, Sun Q, Wang D, Adair KR, Liang J, Zhang C, Zhang L, Lu S, Huang H, Song X, Sun X (2019) Insight into the microstructure and ionic conductivity of cold sintered NASICON solid electrolyte for solid-state batteries. ACS Appl Mater Interfaces 11(31):27890–27896. https://doi.org/10.1021/acsami.9b08132
Wu XM, Liu JL, Chen S, Ma MY, Liu JB (2010) Effect of sintering conditions on the properties of sol-gel-derived Li1.3Al0.3Ti1.7(PO4)3. Ionics 16(9):827–831. https://doi.org/10.1007/s11581-010-0471-9
Funding
Authors thank the financial supports from the National Natural Science Foundation of China (21673131) and from the Taishan Scholar Project of Shandong Province (ts201511004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Q., Zhang, X., Zhang, Y. et al. Influence of lithium phosphate on the structural and lithium-ion conducting properties of lithium aluminum titanium phosphate pellets. Ionics 27, 2473–2481 (2021). https://doi.org/10.1007/s11581-021-04011-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04011-2