Abstract
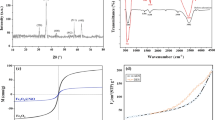
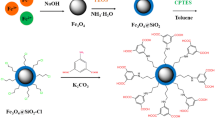
A novel magnetic nano adsorbent based on L-arginine anchored on nano magnetic Fe3O4 particles (MNPs-L) was prepared for removing methyl orange (MO) from aqueous solution. FT-IR and TGA results showed that L-arginine had been modified on the surface of nano magnetic Fe3O4 particles. Influences of many factors on adsorption process were studied, including ionic strength, contact time, initial concentration, pH, and temperature. Kinetics data was found to be followed by pseudo-second-order model. Boyd model suggested that external mass transfer was worse than intraparticle diffusion at rate control for MO adsorption onto MNPs-L. Langmuir model has a full explanation of isotherm data with predicted maximum adsorption capacity for MO to be 149.32 mg/g at 293 K. Moreover, the regeneration assays indicated that MNPs-L had a good stability in recycling. Hence, MNPs-L was a potential adsorbent for removing MO from wastewater.









Similar content being viewed by others
References
Pokhrel D, Viraraghavan T (2004) Treatment of pulp and paper mill wastewater a review. Sci Total Environ 333:37–58
Rai HS, Bhattacharyya MS, Singh J (2005) Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment. Crit Rev Environ Sci Technol 35:219–238
He Y, Jiang DB, Chen J (2018) Evaluation of MnO2-templated iron oxide-coated diatomites for their catalytic performance in heterogeneous photo Fenton-like system. J Hazard Mater 344:230–240
Koupaie EH, Alavi Moghaddam MR, Hashemi SH (2011) Post-treatment of anaerobically degraded azo dye Acid Red 18 using aerobic moving bed biofilm process: enhanced removal of aromatic amines. J Hazard Mater 195:147–154
Alventosa-deLara E, Barredo-Damas S, Alcaina-Miranda MI (2012) Ultrafiltration technology with a ceramic membrane for reactive dye removal: optimization of membrane performance. J Hazard Mater 209-210:492–500
García-Montaño J, Domènech X, García-Hortal JA (2008) The testing of several biological and chemical coupled treatments for Cibacron Red FN-R azo dye removal. J Hazard Mater 154:484–490
Zhang G, Yi L, Deng H (2014) Dyes adsorption using a synthetic carboxymethyl cellulose-acrylic acid adsorbent. J Environ Sci 26:1203–1211
örbahti BK, Artut K, Geçgel C (2011) Electrochemical decolorization of textile dyes and removal of metal ions from textile dye and metal ion binary mixtures. Chem Eng J 173:677–688
Salam MA, Zhrani GA (2012) Simultaneous removal of copper (II), lead(II), zinc(II) and cadmium (II) from aqueous solutions by multi-walled carbon nanotubes. Comptes Rendus Chimie 15:398–408
Liu DG, Li ZH (2013) Adsorption behavior of heavy metal ions from aqueous solution by soy protein hollow microspheres. Ind Eng Chem Res 52:11036–11044
Dong ZH, Zhang F (2015) Polydopamine-mediated surface-functionalization of graphene oxide for heavy metal ions removal. J Solid State Chem 224:88–93
Rahmani A, Zavvar Mousavi H (2010) Effect of nanostructure alumina on adsorption of heavy metals. Desalination 253:94–100
Ayoub A, Richard A (2013) Novel hemicellulose−chitosan biosorbent for water desalination and heavy metal removal. Sustain Chem Eng 1:1102–1109
Wang P, Du ML (2015) Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism. J Hazard Mater 286:533–544
Gao Y, Wu KL, Li HY, Chen W, Fu M, Yue K, Zhu XX, Liu QY (2018) Glutathione detection based on peroxidase-like activity of Co3O4-montmorillonite nanocomposites. Sensors Actuators B 273:1635–1639
Zhu XX, Chen W, Wu KL, Li HY, Fu M, Liu QY, Zhang X (2018) A colorimetric sensor of H2O2 based on Co3O4-montmorillonite nanocomposites with peroxidase activity. New J Chem 42:1501–1509
Guo SZ, Wu KL, Gao Y, Liu LH, Zhu XX, Li XL, Zhang F (2018) Efficient removal of Zn (II), Pb (II), and Cd (II) in waste water based on magnetic graphitic carbon nitride materials with enhanced adsorption capacity. J Chem Eng Data 63:3902–3912
Guo SZ, Jiao PP, Dan ZG et al (2017) Preparation of L-arginine modified magnetic adsorbent by one-step method for removal of Zn(II) and Cd(II) from aqueous solution. Chem Eng J 317:999–1011
Ma J, Zhu ZL, Chen B (2013) One-pot, large-scale synthesis of magnetic activated carbon nanotubes and their applications for arsenic removal. J Mater Chem A 1:4662–4666
Fan LR, Song JQ, Bai WB (2016) Chelating capture and magnetic removal of non-magnetic heavy metal substances from soil. Sci Rep 6:21027
Ma ZY, Guan YP, Liu HZ (2005) Synthesis and characterization of micron-sized mono disperse super paramagnetic polymer particles with amino groups. J Polym Sci A Polym Chem 43:3433–3439
Wang LX, Li JC, Wang YQ (2011) Preparation of nanocrystalline Fe3_xLaxO4 ferrite and their adsorption capability for Congo red. J Hazard Mater 196:342–349
Zhu HY, Jiang R, Xiao L (2010) A novel magnetically separable c-Fe2O3/crosslinked chitosan adsorbent: preparation, characterization and adsorption application for removal of hazardous azo dye. J Hazard Mater 179:251–257
Liu XY, An SA, Wang YJ (2015) Rapid selective separation and recovery of a specific target dye from mixture consisted of different dyes by magnetic Ca-ferrites nanoparticles. Chem Eng J 262:517–526
Han RP, Zhang JJ (2009) Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem Eng J 145:496–504
Huang RH, Liu Q, Huo J (2013) Adsorption of methyl orange onto pro-tonated cross-linked chitosan. Arab J Chem 5:1–8
Jiang T, Liang YD (2015) Activated carbon/NiFe2O4 magnetic composite: a magnetic adsorbent for the adsorption of methyl orange. J Environ Chem Eng 3:1740–1751
He Y, Jiang DB, Chen J (2018) Synthesis of MnO2 nanosheets on montmorillonite for oxidative degradation and adsorption of methylene blue. J Colloid Interface Sci 510:207–220
Ma J, Yu F, Zhou L (2012) Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. Appl Mater Interfaces 4(11):5749–5760
Ren Y, Abbood HA, He F (2013) Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption. Chem Eng J 226:300–311
Gupta VK, Moradi O, Tyagi I (2016) Study on the removal of heavy metal ions from industry waste by carbon nanotubes: effect of the surface modification: a review. Crit Rev Environ Sci Technol 46(2):93–118
Ren X, Chen C, Nagatsu M (2011) Carbon nanotubes as adsorbents in environmental pollution management: a review. Chem Eng J 170:395–410
Masternak J, Machnik MZ, Kowalik M (2016) Recent advances in coordination chemistry of metal complexes based on nitrogen heteroaromatic alcohols. Synthesis, structures and potential applications. Coord Chem Rev 327-328:242–270
Luo X, Zhang L (2009) High effective adsorption of organic dyes on magnetic cellulose beads entrapping activated carbon. J Hazard Mater 171:340–347
Zhu HY, Jiang R, Fu YQ (2011) Preparation, characterization and dye adsorption properties of YFe2O3/ SiO2/chitosan composite. Appl Surf Sci 258:1337–1344
Wu D, Zheng P, Chang PR (2011) Preparation and characterization of magnetic rectorite/iron oxide nanocomposites and its application for the removal of the dyes. Chem Eng J 174:489–494
Istratie R, Stoia M (2015) Single and simultaneous adsorption of methyl orange and phenol onto magnetic iron oxide/carbon nanocomposites. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.12.012
Chen H, Zhao J, Wu J (2011) Isotherm, thermodynamic, kinetics and adsorption mechanism studies of methyl orange by surfactant modified silkworm exuviae. J Hazard Mater 192:246–254
Xie Y, Qian D, Wu D (2011) Magnetic halloysite nanotubes/iron oxide composites for the adsorption of dyes. Chem Eng J 168:959–963
Rad MS, Irandoust M, Amri S (2014) Magnetic solid phase adsorption, preconcentration and determination of methyl orange in water samples using silica coated magnetic nanoparticles and central composite design. Int Nano Lett 4:91–101
Funding
This work was supported by the Natural Science Foundation of Shandong Province (Grant No. ZR2018PEE006), Scientific Research Foundation of Shandong University of Science and Technology for Recruited Talents (Grant No. 2015RCJJ018), and Qingdao Postdoctoral Application Research Project Funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 5497 kb)
Rights and permissions
About this article
Cite this article
Guo, S., Zhu, X., Yang, C. et al. Synthesis and characterization of L-arginine/Fe3O4 adsorbent for the removal of methyl orange from aqueous solutions. Ionics 25, 1323–1330 (2019). https://doi.org/10.1007/s11581-019-02844-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-02844-6