Abstract
The layered structure of LiNi1/3Mn1/3Co1/3 – xYxO2 with different concentration of yttrium substituted (x = 0.00, 0.01, 0.03, and 0.05) cathode material was synthesized using the sol-gel method, followed by high-temperature calcination process. The influence of Y substitution on the microstructure and electrochemical performances of the prepared materials were investigated using X-ray diffraction (XRD), transmission electron microscopy (TEM), cyclic voltammetry and galvanostatic charge/discharge test respectively. The XRD result showed that the LiNi1/3Mn1/3Co1/3 − xYxO2 has a well-ordered hexagonal α-NaFeO2 structure with high crystallinity. The surface morphology of pristine material and Y-doped electrode material revealed the spherical-shaped particles which are uniformly distributed. The cyclic voltammograms of the proposed cathode material also yield a well-defined redox peaks at their corresponding potentials. As the doping of yttrium increases, the discharge capacity also increases attributed to the superior capacity retention at high voltage (4.2 V) even after 100 cycles. Therefore, this composition is a promising cathode material for lithium-ion batteries.

Graphical abstract
Similar content being viewed by others
Introduction
During the past few decades, lithium-ion batteries have garnered enormous attraction among the research community owing to its superior properties such as high energy density, low self-discharge, and even operating at high voltage. However, the issues related to the battery performance like power density, safety, and long cycle life impede their wide applications for commercial purposes [1, 2]. The lithium-ion batteries are widely available for portable consumer applications such as laptops and cellular phones. Lithium-ion batteries are typically constituted of anode, cathode, and porous membrane called separator immersed in a nonaqueous solution are under consideration for the electric vehicle and hybrid electric vehicle applications. The technological challenges confronting their scale-up and commercialization are the capacity fade characteristics and the thermal safety. The electrochemical reaction involved in LIBs is redox reactions from the redox active species, intercalation/deintercalation from a solid matrix material. A significant challenge of lithium-ion battery production is to find out a proper cathode material as an attractive candidate for next-generation cathode materials to replace LiCoO2. Although LiCoO2 is the predominant cathode material, its high cost and toxicity limit its further development. Recently, several other positive electrode materials, such as LiNiO2, LiMn2O4, LiNi1/3Co1/3Mn1/3O2, and LiFePO4, have been reported [3, 4]. Layer-structured LiNi1/3Co1/3Mn1/3O2 cathode material has attracted much attention for their integrated features of LiCoO2, LiNiO2, and LiMn2O4 with higher structural stability, higher capacity, lower cost, safety, and so on. It has been found that the predominant oxidation states of Ni, Co, and Mn are + 2, + 3, and + 4, respectively. Ni2+ and Co3+ are electrochemically active for redox reaction. However, Mn4+ is electrochemically inactive to stabilize the crystal structure [5]. The major part of this development is the positive electrode material, with the first commercialized LiCoO2, now overwritten by the stoichiometric amount of LiNi1/3Co1/3Mn1/3O2 and it is Li-rich materials siblings Li1 + zMnyO2 normally appearing as the composites of the cathode materials x Li2MnO3(1 − x) LiMO2, cathode materials [6,7,8,9,10]. The possibility of using cathode electrode material is due to its higher energy density at a reduced cost. In particular, the Li1 + xMO2 material has been already reported with various phases, including as a single phase and as a two-phase system with main lithium metal oxides phase containing nanodomains of the cathode materials of Li2MnO3. Single phase symmetry and stacking faults in the lithium manganese layered structure along the c-axis, 6 as well with monoclinic space group symmetry [11]. Nevertheless, its rate capability and cycling performance are not satisfactory at high current density [12, 13]. The derivation of this study is mainly related to its low electronic conductivity, transitional metal ion dissolution into the electrolyte, and the instability of the surface layer between the active particles and the electrolyte solution [14,15,16,17,18]. Layered structure LiNi1/3Co1/3Mn1/3O2 is considered to be one of the most important alternative cathode materials in terms of operating voltage, capacity, cyclic stability, thermal stability, and material cost. Recently it was found that doping could raise the inferior rate capability of the LiNi1/3Co1/3Mn1/3O2 material [19,20,21,22]. In this work, the phase and structure of pristine cathode materials were studied, Y doped, LiNi1/3Mn1/3Co1/3O2 positive lithium-ion battery electrodes and their evolution during battery cycling and establishing their structure function relationship of the electrode materials. The stoichiometry of LiMnO2 is determined by the assumption that transition metal oxide Ni, Mn, and Co are in the oxidation states of 2+, 3+, and 4+, respectively. The structure electrochemical properties of the powder were investigated using XRD and the charge-discharge studies. Herein we report the sol-gel synthesis of Y-substituted LiNi1/3Mn1/3Co1/3O2 and intensively investigated the crystallinity, rate capability, and electrochemical performance for rechargeable Li-ion batteries. The results inferred that the as-prepared LiNi1/3Mn1/3Co1/3YxO2 material could be a promising cathode material for real-time applications.
Experimental details
Chemicals
All the chemicals are of analytical grade and used without further purification. Lithium nitrate LiNO3, nickel (II) nitrate (Ni(NO3)2, cobalt (II) nitrate hexahydrate, manganese (II) nitrate and samarium (III) nitrate hexahydrate chemicals and aluminum foil (0.025 mm thickness) were purchased from Alfa Aesar. The solutions are freshly prepared using Millipore water.
Preparation of Y-substituted LiNi1/3Mn1/3Co1/3O2 electrode material
Parent material LiNi1/3Mn1/3Co1/3O2 doped with Y at the following ratio LiNi1/3Mn1/3Co1/3 − xYxO2 (x = 0.00, 0.01, 0.03, 0.05) were synthesized by sol-gel method. Stoichiometric amounts of lithium nitrate anhydrous (LiNO3), nickel (II) nitrate (Ni(NO3)2, cobalt (II) nitrate hexahydrate (CoNO3)2.6H2O, manganese (II) nitrate (MnN2O6.4H2O), and yttrium (III) nitrate hexahydrate (Y(NO3)3.6H2O were dissolved in deionized water and citric acid was added as a chelating agent and continuously stirred. Ammonium hydroxide (NH4OH) was added to maintain the pH value. The gel was formed after 8 h stirring. The obtained gel was dried at 120 °C in a hot air oven to obtain the material in the form of powder. These powders were calcined in a muffle furnace at 800 °C for 10 h. The calcined powders were thoroughly ground in a mortar for further use.
Electrode fabrication
Doctor blade coating method was used to prepare the LiNi1/3Mn1/3Co1/3 − xYxO2 cathode material, with a ratio of 80:10:10. All the active materials were mixed in the proportion of 80 wt% of active materials, 10 wt% of conductive acetylene black, and 10 wt% of PVDF binder dissolved in n-methyl 2-pyrrolidone (NMP) solvent. This product was then applied to the aluminum foil current collector and dried at 120 °C in a hot air oven for 12 h. The electrochemical properties of the 2032 coin cells were assembled in an argon-filled glove box, using the prepared electrodes, lithium foil as the counter electrode, 1 M LiPF6 dissolved in ethyl carbonate and dimethyl carbonate as an electrolyte. Charge/discharge experiments were performed at different current densities between 2.5 and 4.2 V at room temperature. The assembled cells were tested for 100 cycles at a current density of 0.1 C to analyze the cycling performances of the cathode materials.
Characterization
The surface morphology and the crystallite size were examined from scanning electron microscopy and transmission electron microscopy (TEM). The preferred orientation and the crystal structure of the as-prepared battery material were investigated from X-ray diffraction pattern. The elemental composition was ascertained by EDX analysis. The Rct values were obtained from impedance spectroscopy. The electrochemical performances of the LiNi1/3Mn1/3Co1/3O2 and Y-doped samples were explored from cyclic voltammetry. The discharge capacity of LiNi1/3Mn1/3Co1/3O2 and Y-doped samples has analyzed using charge discharge curves.
Results and discussion
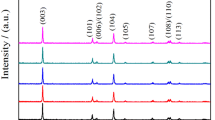
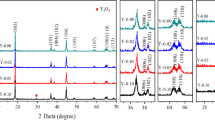
The crystal structure and the preferred orientation were ascertained from XRD patterns. Figure 1 shows the XRD pattern of LiNi1/3Mn1/3Co1/3O2 and doped samples. As seen in Fig. 1a, the peak positioned at 2θ = 18.8, 44.3 and 36.3 are corresponding to the (003), (104), (101) planes of LiNi1/3Mn1/3Co1/3O2 and the preferred orientation is being at (104) plane. The doping of yttrium does not change the orientation. As seen in all the four samples, peak splitting of (006)/(102) and (108)/(110) are observed indicating the formation of layered LiNi1/3Mn1/3Co1/3 − xYxO2 cathode material at all calcination temperatures with respect to the ratio of doping material. However, the weak intense peaks at 43.20 and 63.40 indicate the cubic structured phase of LiNiO2. For all the samples, the diffraction peaks were sharp and well defined suggested the good crystallinity and the crystal structure was identified as a hexagonal α-NaFeO2 structure with R3m space group that confirms the presence of alternative layers of Li, Ni, Mn, and Co in a single-phase layered structure. The peak at 29.2 is due to the formation of Y2O3 which is shown in Fig. 1b, c, and d.
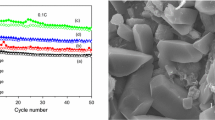
Figure 2 shows the surface morphology of the pristine sample and Y-doped material (x = 0.01, 0.03, 0.05). As depicted in Fig. 2a, the surface of LiNi1/3Mn1/3Co1/3O2 was exhibited as agglomerated spherical-shaped particles with irregular boundaries. In some sites, the hexagon-like particles appeared. As could be observed in Fig. 2b, the morphology of Y-doped sample (x = 0.01) was exhibited as similar spherical-shaped particles and the hexagons are also randomly seen which suggests that the Y doping does not influence much on the surface morphology, but the incorporation of yttrium in the pristine materials greatly enhances the crystallinity which is also reflected in XRD patterns. There is no apparent change in the morphology as x increases which is shown in Fig. 2c and d. However, an increase of dopant concentration leads to the formation of smoother and more compact surface than that of the undoped material.
The elemental composition of the pristine sample and Y-doped sample (x = 0.05) was investigated from EDX analysis and is shown in Fig. 3. The pristine material does not show Y element intensity in EDX spectrum. But for LiNi1/3Mn1/3Co1/3 − xY0.05O2, the high-intense Y peaks confirm the successful doping of yttrium.
Figure 4 reveals the TEM images of parent cathode material and x = 0.05 of Y-doped LiNi1/3Mn1/3Co1/3 − xY0.05O2 cathode heated at 800 °C. As shown in Fig. 4a, the particle size of the sample was estimated as 200 nm. The darkened particles in Fig. 4b indicate that the well-crystallized sample was obtained. In addition, each particle is considered to be a single crystal. There is a thin layer found on the edges of the LiNi1/3Mn1/3Co1/3 − xYxO2 after the doping treatment, where the selected area-diffraction pattern also confirms the single crystalline nature of the material.
Electrochemical characterizations
Cylic voltammetric studies
Cyclic voltammograms of Y-doped LiNi1/3Mn1/3Co1/3 − xYxO2 electrodes are shown in Fig. 5. The curve between first and second does not have a drastic change in the current density values suggesting that the doping of yttrium does not influence the electrochemical behavior. The other reason for the difference of the curve on the first and second cycles of the doped sample was almost unchanged, which means the activation of the electrode on contact with the electrolyte and irreversible loss of capacity is greatly suppressed. The cathodic peak for the pristine sample in the cycle centered at 3.79 V with a current density of 0.0076 A and the corresponding reverse peak centered at 3.72 V is shown in Fig. 5. Subsequently, the anodic peak of the doped sample x = 0.01 centered at 3.79 V and the cathodic peak at 3.71 V with current density values of 0.0077 A and − 0.005 A respectively which is shown as the second cycle in Fig. 5. The CV curve of the Y-doped sample x = 0.03 had a positive peak at 3.80 V with an appreciable current increase from 0.0076 A to 0.0077 A. When compared to the pristine sample, LiNi1/3Mn1/3Co1/3 − xY0.05O2 exhibited well-defined cyclic voltammograms with very high current density values of 0.0083 A at 3.80 V confirms the presence of yttrium in the pristine sample enhances the electrochemical performance of the electrode material. The difference of position between oxidation and reduction peaks of the doping sample was reduced which indicates the better reversibility of Li+ ions.
The electrochemical Nyquist diagrams are shown in Fig. 6. The impedance spectra consist of two semicircles in high and intermediate frequency ranges and a line tending at a constant angle to the original axis in the low-frequency range. The two semicircles in the higher and intermediate frequency range might be due to the contact resistance at the electrode materials and the charge-transfer reaction at the interface of the cathode and electrolyte. The inclined line in the lower frequency range is accredited to Warburg impedance which is associated with Li-ion diffusion through the cathode as observed in Fig. 6, the resistance of LiNi1/3Mn1/3Co1/3 −xY0.05O2 with x = 0.05 is much smaller than that of the undoped electrode. The difference of Rct between charge and discharge was about 148 Ω for the Y-doped electrode, whereas that of undoped one was 222 Ω. The decrease of Rct between charge and discharge is considered to be the effect of yttrium substitution and the electric conductivity increased.
Figure 7 shows the cycle number vs specific capacity profiles of the pristine sample and LiNi1/3Mn1/3Co1/3 − xYxO2 (x = 0, 0.01, 0.03, and 0.05) at 0.1 C rate in room temperature. During the charging process, the charge plateau started at about 3.0 V and slowly increased to about 4.2 V for all samples. The LiNi1/3Mn1/3Co1/3O2 material has delivered the initial discharge capacity of 174.6 mAh g−1and Y-doped LiNi1/3Mn1/3CoO2 exhibited the discharge capacity of 187.3, 208.4, and 227.2 mAh g−1respectively. This clearly suggests that Y-doped LiNi1/3Mn1/3CoO2 showed higher discharge capacities than the parent compound. At the rate of 0.1 C in the voltage, range is from 3.0 to 4.2 V at room temperature. It clearly shows that hexagonal single crystalline LiNi1/3Mn1/3Co1/3 − xY0.05O2 electrode material exhibited better electrochemical performance than that of parent sample.
Figure 8 shows the cycling performance of the LiNi1/3Mn1/3Co1/3O2 and Y-doped LiNi1/3Mn1/3Co1/3O2 samples calcined at 800 °C. When cycled at 0.1 C rate, the Y-doped LiNi1/3Mn1/3Co1/3O2 sample exhibits an initial discharge capacity of 221.9 mAh g−1, which is higher than that of the undoped LiNi1/3Mn1/3Co1/3O2 sample which delivered 164.2 mAh g−1. Even after 100 cycles, the Y-doped LiNi1/3Mn1/3Co1/3O2 sample still delivers 212.6 mAh g−1, retaining 91.3% of its initial discharge capacity. But, for the undoped LiNi1/3Mn1/3Co1/3O2 electrode, the discharge capacity fades after 100 cycles and the capacity retention is 160.4 mAh g−1. The capacity fading is due to the increase in the ohmic drop during the prolonged cycling. The other reason could be the dissolution of the electrode material. As shown in Fig. 7, when x = 0.05, electrode exhibits a higher discharge capacity and better cycle stability than that of the other electrodes at 0.1 C current rate. The data thus demonstrate that the proper yttrium substitution in electrode material improves the cycle stability of the cathode materials at high rates. More interestingly, the cycling stability of as prepared hexagonal LiNi1/3Mn1/3Co1/3 − xY0.05O2 the better than previously reported LiNi1/3Mn1/3Co1/3O2 parent materials. Based on the above results, it is clear that the doped (x = 0.05) materials deliver high initial capacity and excellent cycling stability. The better rate capability of Y-substituted (x = 0.05) electrodes is due to its small particles size and large specific surface area. The overall improved electrochemical characteristics may be attributed to the compositional homogeneity and higher structural stability of the formation of hexagonal single crystals and the high crystallinity of powders may be another important factor that enhances the electrodes specific capacity.
Conclusions
In summary, yttrium-doped LiNi1/3Mn1/3Co1/3O2 has been successfully synthesized by citric acid-assisted sol-gel method. The effects of yttrium-doped layered metal oxide LiNi1/3Mn1/3Co1/3O2 electrode material on battery performance and structural evolution were examined. The structural characterization of Y doping occurs in the main layered R3m phase, with Y doping at the 3a transition metal site and significantly affecting the lattice. The improvement of cycle stability and rate capability can be attributed to the yttrium doping enhances the conductivity and favoring the future use of this cathode electrode materials for high-power applications.
References
Prakash S (2017) Conducting polymers/inorganic nanohybrids for energy applications. Springer International Publishing, Cham, p 365–417
Mohan P, Ranjith B, Kalaignan GP (2014) Structure and electrochemical performances of co-substituted LiSm x La0.2-x Mn1.80O4 cathode materials for rechargeable lithium-ion batteries. J Solid State Electrochem 18:2183–2192
Jiang Q, Li Z, Wang S, Zhang H (2015) A separator modified by high efficiency oxygen plasma for lithium-ion batteries with superior performance. RSC Adv 5:92995–93001
Shi Y, Wang JZ, Chou SL, Wexler D, Li HJ, Ozawa K, Liu HK, Wu YP (2013) Hollow structured Li3VO4 wrapped with graphene nanosheets in situ prepared by a one-pot template-free method as an anode for lithium-ion batteries. Nano Letters 13:4715–4720
Shaju KM, Subba Rao GV, Chowdari BVR (2002) Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries. Electrochim Acta 48:145–151
Chen J, Cheng F (2009) Combination of lightweight elements and nanostructured materials for batteries. Acc Chem Res 42:713–723
Zhu Z, Zhang D, Yan H, Li W (2013) Preparation of high performance spherical hierarchical LiNi0.5Mn1.5O4 for 5 V lithium ion secondary batteries. J Mater Chem A 1:5492–5496
Zhu Y, Gao T, Fan X, Han F, Wang C (2017) Electrochemical techniques for intercalation electrode materials in rechargeable batteries. Acc Chem Res 50:1022–1031
Toprakci O, Toprakci HA, Li Y, Ji L, Xue L, Lee H, Zhang S, Zhang X (2013) Synthesis and characterization of xLi2MnO3(1 − x) LiMn1/3Ni1/3Co1/3O2 composite cathode materials for rechargeable lithium-ion batteries. J Power Sources 241:522–528
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Challenges in the development of advanced Li-ion batteries: a review. Energy Environ Sci 4:3243–3262
Li LJ, Li XH, Wang ZX, Ling WU, Zheng JC, Li JH (2010) Synthesis of LiNi0. 8Co0. 1Mn0.1O2 cathode material by chloride co-precipitation method. Trans Nonferrous Metals Soc China 20:279–282
Zaghib K, Mauger A, Groult H, Goodenough J, Julien C (2013) Advanced electrodes for high power Li-ion batteries. Materials 6:1028–1049
Yan J, Liu H, Wang Y, Zhao X, Mi Y, Xia B (2014) Enhanced high-temperature cycling stability of LiMn2O4 by LiCoO2 coating as cathode material for lithium ion batteries. J Mater Sci Chem Eng 2(12):18
Wang Z, Wang Z, Peng W, Guo H, Li X, Wang J, Qi A (2014) Structure and electrochemical performance of LiCoO2 cathode material in different voltage ranges. Ionics 20:1525–1534
Kong JZ, Ren C, Tai GA, Zhang X, Li AD, Wu D, Li H, Zhou F (2014) Ultrathin ZnO coating for improved electrochemical performance of LiNi0. 5Co0. 2Mn0. 3O2 cathode material. J Power Sources 266:433–439
Wu F, Wang M, Su Y, Chen S (2009) Surface modification of LiCo1/3Ni1/3Mn1/3O2 with Y2O3 for lithium-ion battery. J Power Sources 189:743–747
Huang S, Wilson BE, Wang B, Fang Y, Buffington K, Stein A, Truhlar DG (2015) Y-doped Li8ZrO6 a Li-ion battery cathode material with high capacity. J Am Chem Soc 137:10992–11003
Ding Y, Wang R, Wang L, Cheng K, Zhao Z, Mu D, Wu B (2017) A short review on layered LiNi0.8Co0.1Mn0.1O2 positive electrode material for lithium-ion batteries. Energy Procedia 105:2941–2952
Venkatraman S, Subramanian V, Kumar SG, Renganathan NG, Muniyandi N (2000) Capacity of layered cathode materials for lithium-ion batteries a theoretical study and experimental evaluation. Electrochem Commun 2:18–22
Rao GS, Chowdari BV, Lindner HJ (2001) Yttrium-doped Li (Ni, Co) O2: an improved cathode for Li-ion batteries. J Power Sources 97:313–315
Liu H, Cao Q, Fu LJ, Li C, Wu YP, Wu HQ (2006) Doping effects of zinc on LiFePO4 cathode material for lithium ion batteries. Electrochem Commun 8(10):1553–1557
Feng S, Kong X, Sun H, Wang B, He Y, Liu G (2018) Effect of temperature on Li-rich layered cathode material 0.5Li2MnO3·0.5LiNi0.5Mn0.5O2 prepared by a low temperature solution combustion synthesis. Int J Electrochem Sci 13:9858–9867
Acknowledgements
The first author would like to thank the Department of Science and Technology (DST), New Delhi, for providing financial assistance under DST-INSPIRE Program.
Funding
The authors also thank the DST–PURSE–II and MHRD–RUSA–2.0, New Delhi, for providing financial assistance to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalaiselvi, K., Kalaignan, G.P. Yttrium-substituted LiNi0.3Mn0.3Co0.3O2 cathode material with enhanced cycling stability for rechargeable lithium-ion batteries. Ionics 25, 991–997 (2019). https://doi.org/10.1007/s11581-018-2754-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2754-5