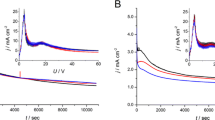
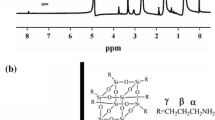
Abstract
In this study, polyaniline (PANi) nanostructures synthesized through a vast majority of straightforward chemical oxidative methods to compare capacitance and rate capability of the products obtained using inexpensive, scalable, and well-established procedures. Effects of deployed method and conditions are assessed on the chemical and pseudocapacitance properties of PANI in aqueous electrolyte. There was a correlation between morphology and electrochemical performance of the samples and a relatively high specific capacity (901 F g−1 at 10 mV s−1) observed for nanofibrous PANi synthesized by interfacial polymerization at room temperature. Ultrasonic assisted polymerization studies showed that higher temperature and irradiation times convert morphology from nanorod to granule and decrease doping degree. However, by deploying HCl as dopant, electrochemical results were comparable with other methods. For instance, lowest charge transfer resistance value (smallest semicircle) and a relatively high capacitance (704 F g−1 at 10 mV s−1) were determined for PANi sample synthesized via ultrasonic assisted polymerization. On the other hand, p-toluenesulfonic acid was a better dopant for PANi synthesized via rapidly mixed reaction. It was shown that three-like nanostructures with high capacitance are obtainable via a template-free rapidly mixed reaction. Results showed that a specific condition and dopant are optimal for each synthetic route and it is possible to obtain PANi with adequate electrochemical performances and morphology in short reaction times even at room temperature, which is very interesting from the industrial point of view.










Similar content being viewed by others
References
Wang H, Lin J, Shen ZX (2016) Polyaniline (PANi) based electrode materials for energy storage and conversion. J Sci Adv Mater Devices 1:225–255. https://doi.org/10.1016/j.jsamd.2016.08.001
Tang S-J, Wang A-T, Lin S-Y, Huang KY, Yang CC, Yeh JM, Chiu KC (2011) Polymerization of aniline under various concentrations of APS and HCl. Polym J 43:667–675. https://doi.org/10.1038/pj.2011.43
Imani A, Farzi G (2015) Facile route for multi-walled carbon nanotube coating with polyaniline: tubular morphology nanocomposites for supercapacitor applications. J Mater Sci Mater Electron 26:7438–7444. https://doi.org/10.1007/s10854-015-3377-5
Cogal S, Cogal GC, Oksuz AU (2017) Plasma-modified multiwalled carbon nanotubes with polyaniline for glucose biosensor applications. Int J Polym Mater Polym Biomater 67(0):1–8. https://doi.org/10.1080/00914037.2017.1342252
Rajender B, Palaniappan S (2015) Simultaneous oxidation and doping of aniline to polyaniline by oxidative template: electrochemical performance in supercapacitor. Int J Polym Mater Polym Biomater 64:939–945. https://doi.org/10.1080/00914037.2015.1038814
Fedorovskaya EO, Okotrub AV, Bulusheva LG (2012) Supercapacitor performance of aligned carbon nanotube/polyaniline composite depending on the duration of aniline polycondensation. Fullerenes Nanotub Carbon Nanostructures 20:519–522. https://doi.org/10.1080/1536383X.2012.655961
Meng Q, Cai K, Chen Y, Chen L (2017) Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 36:268–285. https://doi.org/10.1016/j.nanoen.2017.04.040
Ravi B, Rajender B, Palaniappan S (2016) Improving the electrochemical performance by sulfonation of polyaniline-graphene-silica composite for high performance supercapacitor. Int J Polym Mater Polym Biomater 65:835–840. https://doi.org/10.1080/00914037.2016.1171221
Mike JF, Lutkenhaus JL (2013) Recent advances in conjugated polymer energy storage. J Polym Sci Part B Polym Phys 51:468–480. https://doi.org/10.1002/polb.23256
Stejskal J, Gilbert RG (2002) Polyaniline. Preparation of a conducting polymer(IUPAC Technical Report). Pure Appl Chem. https://doi.org/10.1351/pac200274050857
Huang W-S, Humphrey BD, MacDiarmid AG (1986) Polyaniline, a novel conducting polymer. Morphology and chemistry of its oxidation and reduction in aqueous electrolytes. J Chem Soc Faraday Trans 1 Phys Chem Condens Phases 82:2385. https://doi.org/10.1039/f19868202385
Simotwo SK, Kalra V (2016) Polyaniline-based electrodes: recent application in supercapacitors and next generation rechargeable batteries. Curr Opin Chem Eng 13:150–160. https://doi.org/10.1016/j.coche.2016.09.001
Ismail YA, Chang J, Shin SR, Mane RS, Han SH, Kim SJ (2009) Hydrogel-assisted polyaniline microfiber as controllable electrochemical Actuatable supercapacitor. J Electrochem Soc 156:A313. https://doi.org/10.1149/1.3077597
Eftekhari A, Li L, Yang Y (2017) Polyaniline supercapacitors. J Power Sources 347:86–107. https://doi.org/10.1016/j.jpowsour.2017.02.054
Kuang H, Cao Q, Wang X, Jing B, Wang Q, Zhou L (2013) Influence of the reaction temperature on polyaniline morphology and evaluation of their performance as supercapacitor electrode. J Appl Polym Sci 130:3753–3758. https://doi.org/10.1002/app.39650
Pouget JP, Jozefowicz ME, Epstein AJ, Tang X, MacDiarmid AG (1991) X-ray structure of polyaniline. Macromolecules 24:779–789. https://doi.org/10.1021/ma00003a022
Tran HD, Li D, Kaner RB (2009) One-dimensional conducting polymer nanostructures: bulk synthesis and applications. Adv Mater 21:1487–1499. https://doi.org/10.1002/adma.200802289
Huang J, Kaner RB (2004) Nanofiber formation in the chemical polymerization of aniline: a mechanistic study. Angew Chem 116:5941–5945
Huang J, Kaner RB (2004) A general chemical route to polyaniline nanofibers. J Am Chem Soc 126:851–855. https://doi.org/10.1021/ja0371754
Xia H, Wang Q (2002) Ultrasonic irradiation: a novel approach to prepare conductive polyaniline/nanocrystalline titanium oxide composites. Chem Mater 14:2158–2165. https://doi.org/10.1021/cm0109591
Dan LI, Huang J, Kaner RB (2009) Polyaniline nanofibers: a unique polymer nanostructure for versatile applications. Acc Chem Res 42:135–145. https://doi.org/10.1021/ar800080n
Deng J, Wang T, Guo J, Liu P (2017) Electrochemical capacity fading of polyaniline electrode in supercapacitor: an XPS analysis. Prog Nat Sci Mater Int 27:257–260. https://doi.org/10.1016/j.pnsc.2017.02.007
Moghadam PN, Khalafy J, Taheri T (2010) Sonochemical synthetic methods to produce functionalized conducting copolymers. Polym Adv Technol 21:235–243. https://doi.org/10.1002/pat.1419
Liu H, Hu XB, Wang JY, Boughton RI (2002) Structure, conductivity, and thermopower of crystalline polyaniline synthesized by the ultrasonic irradiation polymerization method. Macromolecules 35:9414–9419. https://doi.org/10.1021/ma0119326
Stejskal J, Kratochvíl P, Radhakrishnan N (1993) Polyaniline dispersions 2. UV-vis absorption spectra. Synth Met 61:225–231. https://doi.org/10.1016/0379-6779(93)91266-5
Gruger A, Novak A, Régis A, Colomban P (1994) Infrared and Raman study of polyaniline part II: influence of ortho substituents on hydrogen bonding and UV/Vis-near-IR electron charge transfer. J Mol Struct 328:153–167. https://doi.org/10.1016/0022-2860(94)08368-1
Du X-S, Zhou C-F, Wang G-T, Mai Y-W (2008) Novel solid-state and template-free synthesis of branched polyaniline nanofibers. Chem Mater 20:3806–3808
Wang Q, Lei Z, Chen Y, Ouyang Q, Gao P, Qi L, Zhu C, Zhang J (2013) Branched polyaniline/molybdenum oxide organic/inorganic heteronanostructures: synthesis and electromagnetic absorption properties. J Mater Chem A 1:11795–11801
Mahdavi H, Kahriz PK, Gholipour-Ranjbar H, Shahalizade T (2017) Synthesis and performance study of amino functionalized graphene aerogel grafted with polyaniline nanofibers as an efficient supercapacitor material. J Mater Sci Mater Electron 28:4295–4305. https://doi.org/10.1007/s10854-016-6053-5
Genies EM, Lapkowski M, Penneau JF (1988) Cyclic voltammetry of polyanilinez interpretation of the add peak. J Electroanal Chem 249:97–107. https://doi.org/10.1016/0022-0728(88)80351-6
Wang Y, Song Y, Xia Y (2016) Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem Soc Rev 45:5925–5950. https://doi.org/10.1039/C5CS00580A
Dou Y, Wang Y, Tian D et al (2017) Atomically thin Co3O4 nanosheet-coated stainless steel mesh with enhanced capacitive Na+ storage for high-performance sodium-ion batteries. 2D Mater 4:15022. https://doi.org/10.1088/2053-1583/4/1/015022
Gholipour-Ranjbar H, Ganjali MR, Norouzi P, Naderi HR (2016) Synthesis of cross-linked graphene aerogel/Fe2O3 nanocomposite with enhanced supercapacitive performance. Ceram Int 42:12097–12104. https://doi.org/10.1016/j.ceramint.2016.04.140
Dhawale DS, Vinu a, Lokhande CD (2011) Stable nanostructured polyaniline electrode for supercapacitor application. Electrochim Acta 56:9482–9487. https://doi.org/10.1016/j.electacta.2011.08.042
Wang K, Wu H, Meng Y, Wei Z (2014) Conducting polymer nanowire arrays for high performance supercapacitors. Small 10:14–31. https://doi.org/10.1002/smll.201301991
Sivaraman P, Kushwaha RK, Shashidhara K, Hande VR, Thakur AP, Samui AB, Khandpekar MM (2010) All solid supercapacitor based on polyaniline and crosslinked sulfonated poly[ether ketone]. Electrochim Acta 55:2451–2456. https://doi.org/10.1016/j.electacta.2009.12.009
Bian LJ, Luan F, Liu SS, Liu XX (2012) Self-doped polyaniline on functionalized carbon cloth as electroactive materials for supercapacitor. Electrochim Acta 64:17–22. https://doi.org/10.1016/j.electacta.2011.12.012
Kim BC, Kwon JS, Ko JM, Park JH, Too CO, Wallace GG (2010) Preparation and enhanced stability of flexible supercapacitor prepared from Nafion/polyaniline nanofiber. Synth Met 160:94–98. https://doi.org/10.1016/j.synthmet.2009.10.011
Prasad KR, Munichandraiah N (2002) Electrochemical studies of polyaniline in a gel polymer electrolyte. Electrochem Solid-State Lett 5:A271–A274. https://doi.org/10.1149/1.1516910
Gupta V, Miura N (2005) Electrochemically deposited polyaniline Nanowire’s network. Electrochem Solid-State Lett 8:A630. https://doi.org/10.1149/1.2087207
Dhawale DS, Salunkhe RR, Jamadade VS, Dubal DP, Pawar SM, Lokhande CD (2010) Hydrophilic polyaniline nanofibrous architecture using electrosynthesis method for supercapacitor application. Curr Appl Phys 10:904–909. https://doi.org/10.1016/j.cap.2009.10.020
Jia R, Wu Y, Zan G, Liu D (2017) Synthesis and characterization of nanotree-like polyaniline electrode material for supercapacitors. J Mater Sci Mater Electron 28:2366–2376
Yoon SB, Yoon EH, Kim KB (2011) Electrochemical properties of leucoemeraldine, emeraldine, and pernigraniline forms of polyaniline/multi-wall carbon nanotube nanocomposites for supercapacitor applications. J Power Sources 196:10791–10797. https://doi.org/10.1016/j.jpowsour.2011.08.107
Mahdavi H, Kahriz PK, Gholipour Ranjbar H, Shahalizade T (2016) Enhancing supercapacitive performance of polyaniline by interfacial copolymerization with melamine. J Mater Sci Mater Electron 27:7407–7414. https://doi.org/10.1007/s10854-016-4715-y
Bian C, Yu A (2010) De-doped polyaniline nanofibres with micropores for high-rate aqueous electrochemical capacitor. Synth Met 160:1579–1583. https://doi.org/10.1016/j.synthmet.2010.04.019
Kampouris DK, Ji X, Randviir EP, Banks CE (2015) A new approach for the improved interpretation of capacitance measurements for materials utilised in energy storage. RSC Adv 5:12782–12791. https://doi.org/10.1039/C4RA17132B
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahdavi, H., Shahalizade, T. Investigation of the pseudocapacitive properties of polyaniline nanostructures obtained from scalable chemical oxidative synthesis routes. Ionics 25, 1331–1340 (2019). https://doi.org/10.1007/s11581-018-2731-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2731-z