Abstract
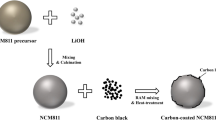
A high-performance Ni/Mo co-doped lithium manganate composite material, LiNi0.03Mo0.01Mn1.96O4, is prepared by a solid-state method, then a biomass-derived carbon layer with ethyl cellulose as the carbon source is applied to the surface of the composite particles. We find that carbon layer with the proper loading can significantly enhance the material’s cyclic stability and capacity at high discharge rates. At rates of 5C and 10C, our optimal sample (LNMMO-3wt%C), with 3 wt% carbon layer loading, has discharge capacities up to 114 and 98 mAh g−1, respectively, which are 10 and 8% higher than those of the uncoated co-doped material. Further, the carbon layer coating significantly improves the material’s stability at high discharge rates: the capacity retention of LNMMO-3wt%C after 400 cycles at discharge rates of 5C and 10C is high reaching 93.6 and 88.1%, respectively, compared with 91.4 and 74.3% for uncoated LNMMO. Based on our experimental results and analysis, we attribute the enhanced stability and capacity at high discharge rates to two factors: (i) enhanced conductivity and (ii) reduced Mn3+ dissolution, combined with significantly decreased resistance from Li+ ion intercalation/de-intercalation, due to the uniformity of the carbon layer coating.

Ni/Mo-doped lithium manganate, LiNi0.03Mo0.01Mn1.96O4, is coated with carbon layer by using biomass-based feedstock ethyl cellulose (EC) as carbon source. The carbon layer coating results significantly improved stability at high discharging rates: the capacity retention of our optimal sample (LNMMO-3wt%C) material after 400 cycles at discharging rates of 5C and 10C are high up to 93.6 and 88.1%, respectively, compared with 91.4 and 74.3% for uncoated material.









Similar content being viewed by others
References
Zhuo HT, Wan S, He CX, Zhang QL, Li CH, Gui DY, Zhu CZ, Niu HB, Liu JH (2014) Improved electrochemical performance of spinel LiMn2O4 in situ coated with graphene-like membrane. J Power Sources 247:721–728. https://doi.org/10.1016/j.jpowsour.2013.09.007
Ding X, Zhou H, Liu G, Yin Z, Jiang Y, Wang X (2015) Electrochemical evaluation of LiAl0.05Ni0.05Mn1.9O4 cathode material synthesized via electrospinning method. J Alloys Compd 632:147–151. https://doi.org/10.1016/j.jallcom.2015.01.163
Reddy MV, Tung BD, Yang L, Quang Minh ND, Loh KP, Chowdari BVR (2013) Molten salt method of preparation and cathodic studies on layered-cathode materials Li(Co0.7Ni0.3)O2 and Li(Ni0.7Co0.3)O2 for Li-ion batteries. J Power Sources 225:374–381. https://doi.org/10.1016/j.jpowsour.2012.07.009
Zhang H-L, Zhang L, Ye L (2014) Synthesis and improvement of Li-Mn spinel by multiple co-doping. Int J Electrochem Sci 9(8):4635–4642
Tian JK, Wan FC, Battaglia VS, Zhang HL (2014) Synthesis and electrochemical performance of nanosized multiple-doped LiMn2O4 prepared at low temperature for Liion battery. Int J Electrochem Sci 9(2):931–942
Guo D, Wei X, Chang Z, Tang H, Li B, Shangguan E, Chang K, Yuan X-Z, Wang H (2015) Synthesis and electrochemical properties of high performance polyhedron sphere like lithium manganese oxide for lithium ion batteries. J Alloys Compd 632:222–228. https://doi.org/10.1016/j.jallcom.2015.01.182
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603. https://doi.org/10.1021/cm901452z
Xia YY, Zhou YH, Yoshio M (1997) Capacity fading on cycling of 4 V Li/LiMn2O4 cells. J Electrochem Soc 144(8):2593–2600. https://doi.org/10.1149/1.1837870
Larcher D, Courjal P, Urbina RH, Gerand B, Blyr A, du Pasquier A, Tarascon JM (1998) Synthesis of MnO2 phases from LiMn2O4 in aqueous acidic media—mechanisms of phase transformations, reactivity, and effect of Bi species. J Electrochem Soc 145(10):3392–3400. https://doi.org/10.1149/1.1838818
Massarotti V, Bini M, Capsoni D, Scardi P, Leoni M (1998) Structure-microstructure relationships in LiMn2O4 spinel phase. Mater Sci Forum 278-281:820–825. https://doi.org/10.4028/www.scientific.net/MSF.278-281.820
Kuriyama K, Onoue A, Yuasa Y, Kushida K (2007) Atomic force microscopy observation of the Jahn-Teller instability in spinel LiMn2O4 embedded in silicon substrates. Aip Conf Proc 893:1481–1482. https://doi.org/10.1063/1.2730467
Guler MO, Akbulut A, Cetinkaya T, Uysal M, Akbulut H (2014) Improvement of electrochemical and structural properties of LiMn2O4 spinel based electrode materials for Li-ion batteries. Int J Hydrog Energy 39(36):21447–21460. https://doi.org/10.1016/j.ijhydene.2014.04.107
Liu Q, Wang S, Tan H, Yang Z, Zeng J (2013) Preparation and doping mode of doped LiMn2O4 for Li-ion batteries. Cheminform 45(5):1718–1730. https://doi.org/10.3390/en6031718
Mateyshina YG, Lafont U, Uvarov NF, Kelder EM (2008) Physical and electrochemical properties of iron-doped lithium–manganese-spinels prepared by different methods. Solid State Ionics 179(1–6):192–196. https://doi.org/10.1016/j.ssi.2007.12.067
Wang FL, Xue HD, Wang K, Liu P, Bai YQ (2013) Summarize the studies on improving the electrochemical performance of spinel LiMn2O4. Adv Mater Res 2013:971–976. https://doi.org/10.4028/www.scientific.net/AMR.690-693.971
Wang MS, Wang J, Zhang J, Fan LZ (2015) Improving electrochemical performance of spherical LiMn2O4 cathode materials for lithium ion batteries by Al-F codoping and AlF3 surface coating. Ionics 21(1):27–35. https://doi.org/10.1007/s11581-014-1164-6
Yu FD, Wang ZB, Chen F, Wu J, Zhang XG, Gu D-M (2014) Crystal structure and multicomponent effects in Li1+xMn2−x−yAlyO4 cathode materials for Li-ion batteries. J Power Sources 262:104–111. https://doi.org/10.1016/j.jpowsour.2014.03.120
Kong L, Li YJ, Li WJ, Li PL, Li HC (2013) Synthesis and characterization of Li1.035Mn1.965O4 and Al-doped Li1.035Al0.035Mn1.930O4 as cathode materials for Li-ion batteries by a wet-chemical technique. J Inorg Mater 28(3):336–340. https://doi.org/10.3724/sp.j.1077.2012.12440
Wang JL, Li ZH, Yang J, Tang JJ, Yu JJ, Nie WB, Lei GT, Xiao QZ (2012) Effect of Al-doping on the electrochemical properties of a three-dimensionally porous lithium manganese oxide for lithium-ion batteries. Electrochim Acta 75(4):115–122. https://doi.org/10.1016/j.electacta.2012.04.136
Xu Y, Chen G, Fu EG, Zhou M, Dunwell M, Fei L, Deng SG, Andersen P, Wang YQ, Jia QX, Luo HM (2013) Nickel substituted LiMn2O4 cathode with durable high-rate capability for Li-ion batteries. RSC Adv 3(40):18441–18445. https://doi.org/10.1039/C3ra42223b
Kebede MA, Kunjuzwa N, Jafta CJ, Mathe MK, Ozoemena KI Kebede MA, Kunjuzwa N, Jafta CJ, Mathe MK, Ozoemena KI (2014) Solution-combustion synthesized nickel-substituted spinel cathode materials (LiNixMn2-xO4; 0≤x≤0.2) for lithium ion battery: enhancing energy storage, capacity retention, and lithium ion transport. Electrochim Acta 128:172–177. https://doi.org/10.1016/j.electacta.2013.11.080
Wang S, Li P, Shao L, Wu K, Lin X, Shui M, Long N, Wang D, Shu J (2015) Preparation of spinel LiNi0.5Mn1.5O4 and Cr-doped LiNi0.5Mn1.5O4 cathode materials by tartaric acid assisted sol–gel method. Ceram Int 41(1):1347–1353. https://doi.org/10.1016/j.ceramint.2014.09.067
Zemin YU, Liancheng Z (2007) Preparation and electrochemical properties of LiMn1.95M0.05O4 (M = Cr, Ni). Rare Metals 26(1):62–67. https://doi.org/10.1016/S1001-0521(07)60029-1
Wang Z, Du J, Li Z, Wu Z (2014) Sol–gel synthesis of co-doped LiMn2O4 with improved high-rate properties for high-temperature lithium batteries. Ceram Int 40(2):3527–3531. https://doi.org/10.1016/j.ceramint.2013.09.076
Yi TF, Chen B, Zhu YR, Li XY, Zhu RS (2014) Enhanced rate performance of molybdenum-doped spinel LiNi0.5Mn1.5O4 cathode materials for lithium ion battery. J Power Sources 247(3):778–785. https://doi.org/10.1016/j.jpowsour.2013.09.031
Suganya Jayapal RM, Piraman S (2013) Dopant depends on morphological and electrochemical characteristics of LiMn2–xMoxO4 cathode nanoparticles. J Solid State Electrochem 17(8):2157–2165. https://doi.org/10.1007/s10008-013-2055-x
Tong QS, Yang Y, Shi JC, Yan JM, Zheng LQ (2007) Synthesis and storage performance of the doped LiMn2O4 spinel. J Electrochem Soc 154(7):A656–A667. https://doi.org/10.1149/1.2731036
Wen W, Ju B, Wang X, Wu C, Shu H, Yang X (2014) Effects of magnesium and fluorine co-doping on the structural and electrochemical performance of the spinel LiMn2O4 cathode materials. Electrochim Acta 147:271–278. https://doi.org/10.1016/j.electacta.2014.09.115
Liu D, He Z, Liu X (2007) Increased cycling stability of AlPO4-coated LiMn2O4 for lithium ion batteries. Mater Lett 61(25):4703–4706. https://doi.org/10.1016/j.matlet.2007.03.012
Tu J, Zhao XB, Xie J, Cao GS, Zhuang DG, Zhu TJ, Tu JP (2007) Enhanced low voltage cycling stability of LiMn2O4 cathode by ZnO coating for lithium ion batteries. J Alloys Compd 432(1–2):313–317. https://doi.org/10.1016/j.jallcom.2006.06.016
Zhao JQ, Wang Y (2013) Atomic layer deposition of epitaxial ZrO2, coating on LiMn2O4, nanoparticles for high-rate lithium ion batteries at elevated temperature. Nano Energy 2(5):882–889. https://doi.org/10.1016/j.nanoen.2013.03.005
Huang B, Li X, Wang Z, Guo H, Xiong X, Wang J (2014) A novel carbamide-assistant hydrothermal process for coating Al2O3 onto LiMn1.5Ni0.5O4 particles used for cathode material of lithium-ion batteries. J Alloys Compd 583(0):313–319. https://doi.org/10.1016/j.jallcom.2013.08.157
Guan D, Jeevarajan JA, Wang Y (2011) Enhanced cycleability of LiMn2O4 cathodes by atomic layer deposition of nanosized-thin Al2O3 coatings. Nanoscale 3(4):1465–1469. https://doi.org/10.1039/c0nr00939c
Ilango PR, Prasanna K, Subburaj T, Jo YN, Lee CW (2015) Facile longitudinal unzipping of carbon nanotubes to graphene nanoribbons and their effects on LiMn2O4 cathodes in rechargeable lithium-ion batteries. Acta Mater 100:11–18. https://doi.org/10.1016/j.actamat.2015.08.021
Qianqian J, Lei X, Zhaoling M, Han Z (2015) Carbon coated to improve the electrochemical properties of LiMn2O4 cathode material synthesized by the novel acetone hydrothermal method. Appl Phys A 119(3):1069–1074. https://doi.org/10.1007/s00339-015-9069-4
Lee S, Cho Y, Song HK, Lee KT, Cho J (2012) Carbon-coated single-crystal LiMn2O4 nanoparticle clusters as cathode material for high-energy and high-power lithium-ion batteries. Angew Chem 51(35):8748–8752. https://doi.org/10.1002/anie.201203581
Zeng J, Li M, Li X, Chen C, Xiong D, Dong L, Li D, Lushington A, Sun X (2014) A novel coating onto LiMn2O4 cathode with increased lithium ion battery performance. Appl Surf Sci 317:884–891. https://doi.org/10.1016/j.apsusc.2014.08.034
Molenda M, Dziembaj R, Podstawka E, Proniewicz LM, Piwowarska Z (2007) An attempt to improve electrical conductivity of the pyrolysed carbon-LiMn2O4−ySy (0 ≤ y ≤ 0.5) composites. J Power Sources 174(2):613–618. https://doi.org/10.1016/j.jpowsour.2007.06.117
Molenda M, Dziembaj R, Piwowarska Z, Drozdek M (2008) Electrochemical properties of C/LiMn2O4 − ySy (0 ≤ y ≤ 0.1) composite cathode materials. Solid State Ionics 179(1–6):88–92. https://doi.org/10.1016/j.ssi.2007.12.033
Zhang H, Zhao H, Zhang Z, Wang W, Liu X (2014) Effects of carbon nanotubes modification on rate capability and high temperature electrochemical performance of LiMn2O4. Electronic Components & Materials 33(5):33–37. https://doi.org/10.3969/j.issn.1001-2028.2014.05.008
Chen M, Chen P, Yang F, Song H, Liao S (2016) Ni, Mo co-doped lithium manganate with significantly enhanced discharge capacity and cycling stability. Electrochim Acta 206:356–365. https://doi.org/10.1016/j.electacta.2016.04.148
Lee JW, Hall AS, Kim J-D, Mallouk TE (2012) A facile and template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability. Chem Mater 24(6):1158–1164. https://doi.org/10.1021/cm203697w
Wang LQ, Jiao LF, Yuan H, Guo J, Zhao M, Li HX, Wang YM (2006) Synthesis and electrochemical properties of Mo-doped Li[Ni1/3Mn1/3Co1/3]O2 cathode materials for Li-ion battery. J Power Sources 162(2):1367–1372. https://doi.org/10.1016/j.jpowsour.2006.08.033
Sinha NN, Munichandraiah N (2009) Synthesis and characterization of carbon-coated LiNi(1/3)Co(1/3)Mn(1/3)O2 in a single step by an inverse microemulsion route. ACS Appl Mater Interfaces 1(6):1241–1249. https://doi.org/10.1021/am900120s
Reddy MV, Sakunthala A, SelvashekaraPandian S, Chowdari BVR (2013) Preparation, comparative energy storage properties, and impedance spectroscopy studies of environmentally friendly cathode, Li(MMn11/6)O4 (M = Mn1/6, Co1/6, (Co1/12Cr1/12)). J Phys Chem C 117(18):9056–9064. https://doi.org/10.1021/jp309180k
Reddy MV, Rao GVS, Chowdari BVR (2011) Nano-(V1/2Sb1/2Sn)O4: a high capacity, high rate anode material for Li-ion batteries. J Mater Chem 21(27):10003–10011. https://doi.org/10.1039/c0jm04140h
Funding
This work was supported by the National Natural Science Foundation of China (NSFC project nos. 21276098, 21476088, 51302091, U1301245), the Department of Science and Technology of Guangdong Province (project nos. 2014A010105041 and 2015B010106012), the Natural Science Foundation of Guangdong Province (project no. 2015A030312007), and the Educational Commission of Guangdong Province (project no. 2013CXZDA003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
1. Biomass-based feedstock ethyl cellulose was found to be an excellent carbon source.
2. The capacity and stability were enhanced significantly by the carbon layer coating.
3. The capacity retention after 400 cycles increased significantly with the carbon layer coating.
Rights and permissions
About this article
Cite this article
Zhang, M., Chen, M., Shao, Y. et al. Enhanced performance of LiNi0.03Mo0.01Mn1.96O4 cathode materials coated with biomass-derived carbon layer. Ionics 25, 917–925 (2019). https://doi.org/10.1007/s11581-018-2608-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2608-1