Abstract
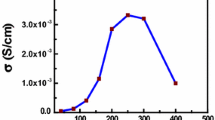
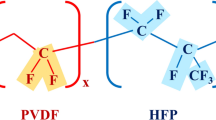
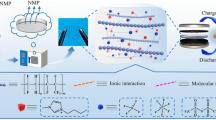
As a polymer host, one polymeric ionic liquid poly(methyl methacrylate-1-vinyl-3-ethyl-imidazolium bis(trifluoromethylsulfonyl) imide) (abbr. P(MMA-co-VEImTFSI)) was successfully synthesized and characterized. Four poly(vinylidene fluoride-co-hexafluoropropylene) (abbr. PVDF-HFP)-based polymer electrolytes were prepared by blending 0, 5,15, and 25 wt% P(MMA-co-VEImTFSI). The electrochemical performances of the prepared electrolytes were studied carefully. The results revealed that increasing the polymeric ionic liquid content, the ionic conductivity of the polymer electrolytes could be enhanced and it obeyed the Arrhenius rule. The highest ionic conductivity of the polymer electrolytes was up to 2.09 × 10−3 S cm−1 at room temperature. The polymer with 25 wt% polymeric ionic liquid showed an excellent electrochemical performance for supercapacitor electrolyte. After 2000 cycles, the retention of capacitance in P(MMA-co-VEImTFSI)-based polymer electrolyte was above 80%. It implied that the present P(MMA-co-VEImTFSI) polymeric ionic liquid was a decent component candidate in the high-performance polymer electrolytes.
















Similar content being viewed by others
References
Anothumakkool B, Torris ATA, Veeliyath S, Vijayakumar V, Badiger MV, Kurungot S (2016) High-performance flexible solid-state supercapacitor with an extended nanoregime interface through in situ polymer electrolyte generation. ACS Appl Mater Interfaces 8:1233–1241
Ahuja P, Sharma RK, Singh G (2015) Solid-state, high-performance supercapacitor using graphene nanoribbons embedded with zinc manganite. J Mater Chem A 3:4931–4937
Wang Q, Song WL, Fan LZ, Shi Q (2015) Effect of polyacrylonitrile on triethylene glycol diacetate-2-propenoic acid butyl ester gel polymer electrolytes with interpenetrating cross linked network for flexible lithium ion batteries. J Power Sources 295:139–148
Liu B, Huang Y, Cao HJ, Zhao L, Huang YX, Song AM, Lin YX, Li X, Wang MS (2017) A novel porous gel polymer electrolyte based on poly (acrylonitrile-polyhedral oligomeric silsesquioxane) with high performances for lithium-ion batteries. J Membr Sci 545:140–149
Wang YY, Hou BH, Guo JZ, Ning QL, Pang WL, Wang J, Lü CL, Wu XL (2018) An ultralong lifespan and low-temperature workable sodium-ion full battery for stationary energy storage. Adv Energy Mater 8:1703252
Guo JZ, Yang Y, Liu DS, Wu XL, Hou BH, Pang WL, Huang KC, Zhang JP, Su ZM (2018) A practicable Li/Na-ion hybrid full battery assembled by a high-voltage cathode and commercial graphite anode: superior energy storage performance and working mechanism. Adv Energy Mater 8:1702504
Hou BH, Wang YY, Guo JZ, Zhang Y, Ning QL, Yang Y, Li WH, Zhang JP, Wang X, Wu XL (2018) A scalable strategy to develop advanced anode for sodium-ion batteries: commercial Fe3O4 derived Fe3O4@ FeS with superior full-cell performance. ACS Appl Mater Interfaces 10:3581–3589
Pandey GP, Hashmi SA (2013) Ionic liquid 1-ethyl-3-methylimidazolium tetracyanoborate-based gel polymer electrolyte for electrochemical capacitors. J Mater Chem A 1:3372–3378
Bao JJ, Tao C, Yu R, Gao MH, Huang YP, Chen CH (2017) Solid polymer electrolyte based on waterborne polyurethane for all-solid-state lithium ion batteries. J Appl Polym Sci 134:45554
Gerbaldi C, Nair JR, Kulandainathan MA, Kumar RS, Ferrara C, Mustarelli P, Stephan AM (2014) Innovative high performing metal organic framework (MOF)-laden nanocomposite polymer electrolytes for all-solid-state lithium batteries. J Mater Chem A 2:9948–9954
Thayumanasundaram S, Rangasamy VS, Seo JW, Locquet JP (2015) Lithium polymer electrolytes based on sulfonated poly (ether ether ketone) for lithium polymer batteries. Eur J Inorg Chem 32:5395–5404
Li LB, Yang XY, Li JS, Xu YP (2018) A novel and shortcut method to prepare ionic liquid gel polymer electrolyte membranes for lithium-ion battery. Ionics 24:735–741
Liu LL, Li ZH, Xia QL, Xiao QZ, Lei GT, Zhou XD (2012) Electrochemical study of P(VDF-HFP)/PMMA blended polymer electrolyte with high-temperature stability for polymer lithium secondary batteries. Ionics 18:275–281
Dong Y, Ding TJ, Fan LZ (2017) A free-standing and thermostable polymer/plastic crystal electrolyte for all-solid-state lithium batteries. Ionics 23:3339–3345
Wang X, Yang CY, Wang GC (2016) Stretchable fluoroelastomer quasi-solid-state organic electrolyte for high-performance asymmetric flexible supercapacitors. J Mater Chem A 4:14839–14848
Huang Y, Zhong M, Shi F, Liu X, Tang Z, Wang Y, Huang Y, Hou H, Xie X, Zhi C (2017) An intrinsically stretchable and compressible supercapacitor containing a polyacrylamide hydrogel electrolyte. Angew Chem Int Ed 56:9141–9145
Zhu M, Huang Y, Huang Y, Li H, Wang Z, Pei Z, Xue Q, Geng H, Zhi C (2017) A highly durable, transferable, and substrate-versatile high-performance all-polymer micro-supercapacitor with plug-and-play function. Adv Mater 29:1605137
Lei Z, Chen B, Koo YM, MacFarlane DR (2017) Introduction: ionic liquids. Chem Rev 117:6633–6635
MacFarlane DR, Forsyth M, Howlett PC, Pringle JM, Sun JZ, Annat G, Neil W, Izgorodina EI (2007) Ionic liquids in electrochemical devices and processes: managing interfacial electrochemistry. Acc Chem Res 40:1165–1173
Qian WJ, Texter J, Yan F (2017) Frontiers in poly(ionic liquid)s: syntheses and applications. Chem Soc Rev 46:1124–1159
Dong T, Zhang SJ, Zhang L, Chen SM, Lu XM (2015) Improving cycling performance of LiMn2O4 battery by adding an ester-functionalized ionic liquid to electrolyte. Aust J Chem 68:1911–1917
Pont AL, Marcilla R, Meatza DI, Grande H, Mecerreyes D (2009) Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes. J Power Sources 188:558–563
Balducci A, Jeong SS, Kim GT, Passerini S, Winter M, Schmuck M, Appetecchi GB, Marcilla R, Mecerreyes D, Barsukov V, Cantero I, Meatza D, Holzapfel M, Tran N (2011) Development of safe, green and high performance ionic liquids-based batteries (ILLIBATT project). J Power Sources 196:9719–9730
Huang KC, Li HH, Fan HH, Guo JZ, Xing YM, Wu XL, Zhang JP (2017) An in-situ-fabricated composite polymer electrolyte containing large-anion lithium salt for all-solid-state LiFePO4/Li batteries. ChemElectroChem 4:1–8
Wu X-L, Li Y-H, Wu N, Xin S, Kim J-H, Yan Y, Lee JS, Guo YG (2013) Enhanced working temperature of PEO-based polymer electrolyte via porous PTFE film as an efficient heat resister. Solid State Ionics 245:1–7
Appetecchi GB, Kim GT, Montanino M, Carewska M, Marcilla R, Mecerreyes D, De Meatza I (2010) Ternary polymer electrolytes containing pyrrolidinium-based polymeric ionic liquids for lithium batteries. J Power Sources 195:3668–3675
Li MT, Yang L, Fang SH, Dong SM, Hirano SI, Tachibana K (2012) Polymerized ionic liquids with guanidinium cations as host for gel polymer electrolytes in lithium metal batteries. Polym Int 61:259–264
Li MT, Yang L, Fang SH, Dong SM, Hirano SI, Tachibana K (2011) Polymer electrolytes containing guanidinium-based polymeric ionic liquids for rechargeable lithium batteries. J Power Sources 196:8662–8668
Li MT, Yang BL, Wang L, Zhang Y, Zhang Z, Fang SH, Zhang ZX (2013) New polymerized ionic liquid (PIL) gel electrolyte membranes based on tetraalkylammonium cations for lithium ion batteries. J Membr Sci 447:222–227
Li MT, Wang L, Yang BL, Du TT, Zhang Y (2014) Facile preparation of polymer electrolytes based on the polymerized ionic liquid poly ((4-vinylbenzyl) trimethylammonium bis(trifluoromethanesulfonylimide)) for lithium secondary batteries. Electrochim Acta 23:296–302
Yin K, Zhang ZX, Yang L, Hirano SI (2014) An imidazolium-based polymerized ionic liquid via novel synthetic strategy as polymer electrolytes for lithium ion batteries. J Power Sources 258:150–154
Yin K, Zhang ZX, Li XW, Yang L, Tachibana K, Hirano SI (2015) Polymer electrolytes based on dicationic polymeric ionic liquids: application in lithium metal batteries. J Mater Chem A 3:170–178
Porcarelli L, Shaplov AS, Salsamendi M, Nair JR, Vygodskii YS, Mecerreyes D, Grbaldi C (2016) Single-ion block copoly(ionic liquid)s as electrolytes for all-solid state lithium batteries. ACS Appl Mater Interfaces 8:10350–10359
Tiruye GA, Munoz-Torrero D, Palma J, Anderson M, Marcilla R (2015) All-solid state supercapacitors operating at 3.5 V by using ionic liquid based polymer electrolytes. J Power Sources 279:472–480
Du CH, Ma XM, Wu CJ, Cai MQ, Hu MX, Wang T (2015) Polymerizable ionic liquid copolymer P(MMA-co-BVIm-Br) and its effect on the surface wettability of PVDF blend membranes. Chin J Polym Sci 33:857–868
Pekel N, Şahiner N, Güven O, Rzaev ZMO (2001) Synthesis and characterization of N-vinylimidazole–ethyl methacrylate copolymers and determination of monomer reactivity ratios. Eurpolym J 37:2443–2451
Kumar R, Sekhon SS (2008) Effect of molecular weight of PMMA on the conductivity and viscosity behavior of polymer gel electrolytes containing NH4CF3SO3. Ionics 14:509–514
Arunkumar R, Babu RS, Usha Rani M, Kalainathan S (2017) Effect of PBMA on PVC-based polymer blend electrolytes. J Appl Polym Sci 134:44939
Pandey GP, Hashmi SA (2013) Performance of solid-state supercapacitors with ionic liquid 1-ethyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate based gel polymer electrolyte and modified MWCNT electrodes. Electrochim Acta 105:333–341
Liu YQ, Weng B, Razal JM, Xu Q, Zhao C, Hou YY, Seyedin SY, Jalili R, Wallace GG, Chen J (2015) High-performance flexible all-solid-state supercapacitor from large free-standing graphene-PEDOT/PSS films. Sci Rep 5:17045
Liew CW, Ramesh S, Arof AK (2015) Characterization of ionic liquid added poly (vinyl alcohol)-based proton conducting polymer electrolytes and electrochemical studies on the supercapacitors. Int J Hydrog Energy 40:852–862
Zhang X, Liu T, Zhang SF, Huang X, Xu BQ, Lin YH, Xu B, Li LL, Nan CW, Shen Y (2017) Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly (vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J Am Chem Soc 139:13779–13785
Liang B, Tang SQ, Jiang QB, Chen CS, Chen X, Li SL, Yan XC (2015) Preparation and characterization of PEO-PMMA polymer composite electrolytes doped with nano-Al2O3. Electrochim Acta 169:334–341
Yang CY, Sun MQ, Wang X, Wang GC (2015) A novel flexible supercapacitor based on cross-linked PVDF-HFP porous organogel electrolyte and carbon nanotube paper@ π-conjugated polymer film electrodes. ACS Sustain Chem Eng 3:2067–2076
Yadav N, Mishra K, Hashmi S (2017) Optimization of porous polymer electrolyte for quasi-solid-state electrical double layer supercapacitor. Electrochim Acta 235:570–582
Zhao K, Qin QQ, Wang HF, Yang Y, Yan J, Jiang XM (2017) Antibacterial triboelectric membrane-based highly-efficient self-charging supercapacitors. Nano Energy 36:30–37
Kim TY, Lee HW, Stoller M, Dreyer DR, Bielawski CW, Ruoff RS, Suh KS (2010) High-performance supercapacitors based on poly (ionic liquid)-modified graphene electrodes. ACS Nano 5:436–442
Zhong XW, Tang J, Cao LJ, Kong WG, Sun Z, Cheng H, Lu ZG, Pan H, Xu BM (2017) Cross-linking of polymer and ionic liquid as high-performance gel electrolyte for flexible solid-state supercapacitors. Electrochim Acta 244:112–118
Vijayakumar V, Anothumakkool B, Torris ATA, Nair SB, Badiger MV, Kurungot S (2017) An all-solid-state-supercapacitor possessing a non-aqueous gel polymer electrolyte prepared using a UV-assisted in situ polymerization strategy. J Mater Chem A 5:8461–8476
Li ZF, Ma GQ, Ge R, Qin F, Dong XY, Meng W, Liu TF, Tong JH, Jiang FY, Zhou YF, Li K, Min X, Huo KF, Zhou YH (2016) Free-standing conducting polymer films for high-performance energy devices. Angew Chem Int Ed 55:979–982
Funding
This work was supported financially by the National Key R&D Program of China (No. 2017YFB0102000), the National Natural Science Foundation of China (91534109, 51561145020), Key Research Program of Frontier Sciences, CAS(QYZDY-SSW-JSC011), CAS/SAFEA International Partnership Program for Creative Research Teams (20140491518), and Science and Technology Open Cooperation Project of Henan province (No. 17210110019).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wen, X., Dong, T., Liu, A. et al. A new solid-state electrolyte based on polymeric ionic liquid for high-performance supercapacitor. Ionics 25, 241–251 (2019). https://doi.org/10.1007/s11581-018-2582-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2582-7