Abstract
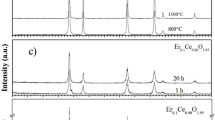
ZrP2O7-CeP2O7 solid solutions, Zr1-xCexP2O7; x = 0–0.2 and (Zr0.92Y0.08)1-yCeyP2O7; y = 0–0.1, were prepared by partially replacing Zr4+ with Ce4+ and its effect on the phase composition, sintering behavior, microstructure, and ionic conductivity is analyzed. Ce4+-doped specimens showed improved sintering behavior due to the partial reduction of Ce4+ to Ce3+, as confirmed by X-ray photoelectron spectroscopy (XPS). In unhumidified atmosphere, the electrical conductivity of Zr1-xCexP2O7 increased with increasing cerium content, which can be attributed to the increase in densification and formation of oxygen vacancies due to the partial reduction of Ce4+ to Ce3+. For (Zr0.92Y0.08)1-yCeyP2O7; y = 0–0.1 specimens, the electrical conductivity increased ≥ 4 orders of magnitude during humidification in air (pH2O = 0.12 atm). At 80 °C, specimen (Zr0.92Y0.08)0.9Ce0.1P2O7 (ZYCP10) showed a maximum of 1.72 × 10−2 S cm−1 which decreased sharply at 100 °C. Furthermore, Zr0.92Y0.08P2O7 (ZYP), (Zr0.92Y0.08)0.95Ce0.05P2O7 (ZYCP5), and ZYCP10 specimens humidified at 160 °C showed the maximum conductivity of 1.04 × 10−3, 1.32 × 10−3, and 8.09 × 10−3 S cm−1, respectively, at 190 °C.








Similar content being viewed by others
References
Wang H, He X, Shi Y, Sun L, Luo C, Cui Y (2015) A H3PO4-doped polytetrafluoroethylene/Sn0.95Mg0.05P2O7 proton-conducting inorganic–organic composite electrolyte. Mater Lett 145:1–3
Wang H, Chen J, Luo C, Qiao R (2014) Electrical conduction of CeP2O7 and Ce0.95Yb0.05P2O7 at intermediate temperatures. Solid State Ionics 263:71–74
Zhang H, Xiao J, Yang Z, Wang H, Ma G, Zhou Z (2012) Ionic conduction in Zn2+-doped ZrP2O7 ceramic at intermediate temperatures. Solid State Ionics 218:1–6
Zhang H, Xiao J, Yang Z, Wang H, Ma G (2012) Ionic conduction in In3+-doped ZrP2O7 at intermediate temperatures. Chin J Chem 30:1826–1830
Zhao D, Deng X, Ding Z, Wang H, Ma G (2012) Intermediate temperature ionic conduction in Mg2+-doped ZrP2O7 ceramics. Solid State Ionics 229:33–37
Singh B, Kim JH, Parkash O, Song SJ (2016) Effect of MnO doping in tetravalent metal pyrophosphate (MP2O7; M=Ce, Sn, Zr) electrolytes. Ceram Int 42:2983–2989
Singh B, Kim JH, Park JY, Song SJ (2015) Effect of partial substitution of Sn4+ by M4+ (M=Si, Ti, and Ce) on sinterability and ionic conductivity of SnP2O7. Ceram Int 41:3339–3343
Kato K, Takenaka T, Takata M, Shinozaki K (2008) Synthesis and lithium ion conductivity of MP2O7-based solid electrolytes. Key Eng Mater 388:53–56
Ide K, Suzuki S, Miyayama M (2010) Synthesis and lithium ion conductivities of zirconium phosphate-based solid electrolytes. Key Eng Mater 445:105–108
Al-Zahrani SM, Elbashir NO, Abasaeed AE, Abdulwahed M (2000) Oxidative dehydrogenation of isobutane over pyrophosphate catalytic systems. Catal Lett 69:65–70
Samed AJ, Uemura K, Ikeue K, Machida M (2013) Catalytic NO–CO–C3H6–O2 reactions over hydrothermally stable Rh-supported ZrP2O7 catalyst. Int J Environ Stud 70:549–559
Marcu IC, Sandulescu I, Millet JMM (2002) Oxidehydrogenation of n-butane over tetravalent metal phosphates based catalysts. App Catal A 227:309–320
Xiang H, Feng Z, Zhou Y (2014) Dynamical and dielectric properties of MP2O7 (M = Ti, Zr, and Hf): a first-principles investigation. Comput Mater Sci 95:371–376
Kim CH, Yim HS (1999) The effect of tetravalent metal on dielectric property in ZrP2O7 and TiP2O7. Solid State Commun 110:137–142
Chen F, Shen Q, Schoenung JM, Zhang L (2008) Synthesis and pressureless sintering of zirconium phosphate ceramics. J Am Ceram Soc 91:3173–3180
Itatani K, Kishioka A, Kinoshita M (1984) Effect of liquid phase on sintering of magnesium oxide with zirconium diphosphate addition. J Ceram Assoc Japan 92:530–533
Ota T, Yamai I (1987) Thermal expansion of ZrP2O7 and related solid solutions. J Mater Sci 22:3762–3764
Watanabe M, Sakurai M, Yamada T, Mori H (1996) Synthesis and thermal properties of zirconium ortho- and diphosphates. J Mater Sci 31:2569–2572
Xiang H, Feng Z, Zhou Y (2014) Ab initio computations of electronic, mechanical, lattice dynamical and thermal properties of ZrP2O7. J Euro Ceram Soc 34:1809–1818
Khosrovani N, Korthuis V, Sleight AW, Vogt T (1996) Unusual 180° P−O−P bond angles in ZrP2O7. Inorg Chem 35:485–489
Stinton GW, Hampson MR, Evans JSO (2006) The 136-atom structure of ZrP2O7 and HfP2O7 from powder diffraction data. Inorg Chem 45:4352–4358
Maârouf I, Oulmekki A, Toyir J, Nawdali M, Zarguili I, El Ghadraoui H (2017) Development of new ZrxTi1-xP2O7 materials as model compounds for the recycling of metallic waste from phosphate chemistry. J Mater Environ Sci 8:2807–2815
Raymond WS (1961) Separation of plutonium ions from solution by adsorption on zirconium pyrophosphate, US Patent 2970035
Pelova V, Grigorov L (1996) Luminescence of copper (I) in zirconium pyrophosphate. J Mater Sci Lett 15:1225–1227
Wu C, Wan Y (2007) Study of excitation spectra of ZrP2O7:Tb in vacuum ultraviolet region. Mater Lett 61:5037–5039
Gorodylova N, Šulcová P, Bosacka M, Filipek E, Vlček M (2015) Heterovalent Zr4+-Cu2+ substitution in zirconium pyrophosphate: from theoretical models to synthesis and utilization. J Eur Ceram Soc 35:4293–4305
Hizhnyi Y, Chornii V, Nedilko S, Slobodyanik M, Zatovsky I, Terebilenko K, Boyko V (2013) Luminescence spectroscopy and electronic structure of ZrP2O7 and KZr2(PO4)3 crystals. Radiat Meas 56:397–401
Nalini V, Amezawa K, Xing W, Norby T (2010) High temperature proton conductivity of ZrP2O7. J Electrochem Soc 157:B1491–B1498
Paschos O, Kunze J, Stimming U, Maglia F (2011) A review on phosphate based, solid state, protonic conductors for intermediate temperature fuel cells. J Phys 23:234110
Phadke SR, Bowers CR, Wachsman ED, Nino JC (2011) Proton conduction in acceptor doped SnP2O7. Solid State Ionics 83:26–31
Singh B, Im HN, Park JY, Song SJ (2012) Electrical behavior of CeP2O7 electrolyte for the application in low temperature proton conducting ceramic electrolyte fuel cells. J Electrochem Soc 159:F819–F825
Shen YB, Nishida M, Kanematsu W, Hibino T (2011) Synthesis and characterization of dense SnP2O7-SnO2 composite ceramics as intermediate-temperature proton conductors. J Mater Chem 21:663–670
Singh B, Kim JH, Park JY, Song SJ (2014) Ionic conductivity of Mn2+ doped dense tin pyrophosphate electrolytes synthesized by a new co-precipitation method. J Eur Ceram Soc 34:2967–2976
Singh B, Kim JH, Park JY, Song SJ (2015) Dense composite electrolytes of Gd3+-doped cerium phosphates for low-temperature proton-conducting ceramic-electrolyte fuel cells. Ceram Int 41:4814–4821
Singh B, Bhardwaj A, Gautam SK, Kumar D, Parkash O, Kim IH, Song SJ (2017) Fast ionic conduction in tetravalent metal pyrophosphate-alkali carbonate composites: new potential electrolytes for intermediate-temperature fuel cells. J Power Sources 354:176–181
Kim JH, Singh B, Hong JW, Im HN, Song SJ (2016) Electrical behavior and stability of K2HPO4-KH5(PO4)2-Ce0.9Gd0.1P2O7 composite electrolytes for intermediate temperature proton-conducting fuel cells. J Electrochem Soc 163:F225–F229
Kim JH, Park EJ, Lim DK, Singh B, Bae C, Song SJ (2015) Fabrication of dense cerium pyrophosphate-polystyrene composite for application as low-temperature proton-conducting electrolytes. J Electrochem Soc 162:F1159–F1164
Le MV, Tsai DS, Yang CY, Chung WH, Lee HY (2011) Proton conductors of cerium pyrophosphate for intermediate temperature fuel cell. Electrochim Acta 56:6654–6660
Singh B, Jeon SY, Kim JH, Park JY, Bae C, Song SJ (2014) Ionic conductivity of Gd3+ doped cerium pyrophosphate electrolytes with core-shell structure. J Electrochem Soc 161:F464–F472
Ryu KH, Haile SM (1999) Chemical stability and proton conductivity of doped BaCeO3-BaZrO3 solid solutions. Solid State Ionics 125:355–367
Katahira K, Kohchi Y, Shimura T, Iwahara H (2000) Protonic conduction in Zr-substituted BaCeO3. Solid State Ionics 138:91–98
Zhong Z, Sayir A, Dynys F (2004) Sintering and stability of the BaCe09-xZrxY01O3-δ system, Ceram Eng Sci Proc 25:345–350, 28th International Conference on Advanced Ceramics and Composites; Cocoa Beach, FL; US
Hirai H, Masui T, Imanaka N, Adachi G (2004) Characterization and thermal behavior of amorphous rare earth phosphates. J Alloys Comp 374:84–88
Zhou Y (1998) The influence of redox reaction of the sintering of cerium oxide. J Mater Synth Process 6:411–414
Foschini CR, Perazolli L, Varela JA (2004) Sintering of tin oxide using zinc oxide as a densification aid. J Mater Sci 39:5825–5830
Huang X, Wang G, Huang M, Deng Y, Fei MM, Xu C, Cheng J (2017) Ce3+ doped CeP2O7 ceramic electrolyte for high temperature proton exchange membrane fuel cell. Int J Electrochem Sci 12:2731–2740
Li Y, Kunitake T, Aoki Y, Muto E (2008) Efficient, anahydrous proton-conducting nanofilms of Y-doped zirconium pyrophosphate at intermediate temperatures. Adv Mater 20:2398–2404
Alberti G, Casciola M, Cavalaglio S, Vivani R (1999) Proton conductivity of mesoporous zirconium phosphate pyrophosphate. Solid State Ionics 125:91–97
Funding
This research was supported by the financial assistance from the Science and Engineering Research Board (SERB), Department of Science and Technology (DST), New Delhi, under the Ramanujan Fellowship (SB/S2/RJN-120/2014), Mid-Career Researcher Program (2015R1A2A2A01003852) through NRF grant funded by the MEST, Republic of Korea, and by “KIST Institutional Program” from the Korea Institute of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 428 kb)
Rights and permissions
About this article
Cite this article
Gautam, S.K., Singh, A., Mathur, L. et al. Sintering and electrical behavior of ZrP2O7–CeP2O7 solid solutions Zr1-xCexP2O7; x = 0–0.2 and (Zr0.92Y0.08)1-yCeyP2O7; y = 0–0.1 for application as electrolyte in intermediate temperature fuel cells. Ionics 25, 155–162 (2019). https://doi.org/10.1007/s11581-018-2563-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2563-x