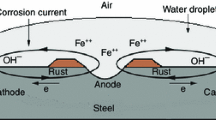
Abstract
This article reported the electrochemical deposition of polyaniline (PANI) on 316-L stainless steel (316LSS) to improve the anti-corrosion performance as PEMFC metal bipolar plates. The results indicate that PANI can increase the corrosion potential of 316LSS by more than 410.57 mV and effectively decrease the corrosion current density by four orders of magnitude in comparison with the uncoated 316LSS. The experimental results showed that the PANI increased the open-circuit potential of the steel by about 140 mV. The polarization current value of PANI-coated 316LSS reduced to 2.3 × 10−7 A/cm2 under the PEMFC cathode working condition. During exposure for 12 h, Nyquist plots of PANI-coated 316LSS did not change substantially. This indicates that the PANI coating was an effective barrier against the inward penetration of corrosive species.










Similar content being viewed by others
References
Lim BH, Majlan EH, Daud WRW, Husaini T, Rosli MI (2016) Effects of flow field design on water management and reactant distribution in PEMFC: a review. Ionics 22(3):301–316
Mehta V, Cooper JS (2003) Review and analysis of PEM fuel cell design and manufacturing. J Power Sources 114(1):32–53
Lee WG, Cho KH, Lee SB, Park SB, Jang H (2009) Electrochemical response of zirconia-coated 316L stainless-steel in a simulated proton exchange membrane fuel cell environment. J Alloys Compd 474(1):268–272
Ren YJ, Chen J, Zeng CL (2010) Corrosion protection of type 304 stainless steel bipolar plates of proton-exchange membrane fuel cells by doped polyaniline coating. J Power Sources 195(7):1914–1919
Cunningham BD, Huang J, Baird DG (2007) Review of materials and processing methods used in the production of bipolar plates for fuel cells. Int Mater Rev 52(1):1–13
Tawfik H, Hung Y, Mahajan D (2007) Metal bipolar plates for PEM fuel cell—a review. J.Power Sources 163(2):755–767
Davies DP, Adcock PL, Turpin M, Rowen SJ (2000) Stainless steel as a bipolar plate material for solid polymer fuel cells. J Power Sources 86(1):237–242
Wang Y, Northwood DO (2006) An investigation into polypyrrole-coated 316L stainless steel as a bipolar plate material for PEM fuel cells. J Sources 163(1):500–508
Ren YJ, Zeng CL (2007) Corrosion protection of 304 stainless steel bipolar plates using TiC films produced by high-energy micro-arc alloying process [J]. J Power Sources 171(2):778–782
Wang SH, Peng J, Lui WB, Zhang JS (2006) Performance of the gold-plated titanium bipolar plates for the light weight PEM fuel cells. J Power Sources 162(1):486–491
Wang SH, Peng J, Lui WB (2006) Surface modification and development of titanium bipolar plates for PEM fuel cells. J Power Sources 160(1):485–489
Joseph S, McClure JC, Sebastian PJ, Moreira J, Valenzuela E (2008) Polyaniline and polypyrrole coatings on aluminum for PEM fuel cell bipolar plates. J Power Sources 177(1):161–166
Hung Y, El-Khatib KM, Tawfik H (2006) Testing and evaluation of aluminum coated bipolar plates of PEM fuel cells operating at 70 C. J Power Sources 163(1):509–513
Nikam VV, Reddy RG (2006) Corrugated bipolar sheets as fuel distributors in PEMFC. Int J Hydrogen Ener 31(13):1863–1873
Hsieh SS, Huang CF, Feng CL (2008) A novel design and micro-fabrication for copper (Cu) electroforming bipolar plates. Micron 39(3):263–268
Wind J, Späh R, Kaiser W, Böhm G (2002) Metallic bipolar plates for PEM fuel cells. J Power Sources 105(2):256–260
Pan TJ, Zuo XW, Wang T, Hu J, Chen ZD, Ren YJ (2016) Electrodeposited conductive polypyrrole/polyaniline composite film for the corrosion protection of copper bipolar plates in proton exchange membrane fuel cells. J Power Sources 302:180–188
Hentall PL, Lakeman JB, Mepsted GO, Adcock PL, Moore JM (1999) New materials for polymer electrolyte membrane fuel cell current collectors. J Power Sources 80(1):235–241
Li M, Luo S, Zeng C, Shen J, Lin H, Cao CN (2004) Corrosion behavior of TiN coated type 316 stainless steel in simulated PEMFC environments. Corros Sci 46(6):1369–1380
Cho EA, Jeon US, Hong SA, Oh IH, Kang SG (2005) Performance of a 1kW-class PEMFC stack using TiN-coated 316 stainless steel bipolar plates. J Power Sources 142(1):177–183
Feng K, Kwok DT, Liu D, Li Z, Cai X, Chu PK (2010) Nitrogen plasma-implanted titanium as bipolar plates in polymer electrolyte membrane fuel cells. J Power Sources 195(19):6798–6804
Show Y, Miki M, Nakamura T (2007) Increased in output power from fuel cell used metal bipolar plate coated with a-C film. Diam Relat Mater 16(4):1159–1161
DeBerry DW (1985) Modification of the electrochemical and corrosion behavior of stainless steels with an electroactive coating. J Electrochem Soc 132(5):1022–1026
Apichartsrangkoon A, Ledward DA (2002) Dynamic viscoelastic behaviour of high pressure treated gluten-soy mixtures. Food Chem 77(3):317–323
Gupta A (2001) Optimization of product performance of a paint formulation using a mixture experiment. J Appl Stat 28(2):199–213
Wen TC, Chen WC (2000) Blending thermoplastic polyurethanes and poly (ethylene oxide) for composite electrolytes via a mixture design approach. J Appl Pol Sci 77(3):680–692
Roberts FL, Mohammad LN, Wang LB (2002) History of hot mix asphalt mixture design in the United States. J Mater Civ Eng 14(4):279–293
Ribeiro AB, De Almeida IR (2000) Study on high performance roller compacted concrete. Mater Struct 33(6):398–402
Piepel G, Redgate T (1997) Mixture experiment techniques for reducing the number of components applied for modeling waste glass sodium release. J Am Ceram Society 80(12):3038–3044
Schabbach LM, Fredel MC, Hotza D (2001) Three-component lead borosilicate frit. Am Ceram Soc Bull 80(7):57–63
Ren YJ, Chen J, Zeng CL, Li C, He JJ (2016) Electrochemical corrosion characteristics of conducting polypyrrole/polyaniline coatings in simulated environments of a proton exchange membrane fuel cell. Int. J. Hydrogen Ener. 41(20):8542–8549
Acknowledgements
This work was financially supported by Beijing’s Key Research Program (no. Z171100000917010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, P., Ding, X., Yang, Z. et al. Electrochemical synthesis and characterization of polyaniline-coated PEMFC metal bipolar plates with improved corrosion resistance. Ionics 24, 1129–1137 (2018). https://doi.org/10.1007/s11581-017-2274-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2274-8