Abstract
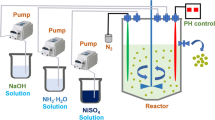
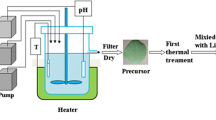
(Ni0.8Mn0.1Co0.1)(OH)2 and Co(OH)2 secondly treated by LiNi0.8Mn0.1Co0.1O2 have been prepared via co-precipitation and high-temperature solid-state reaction. The residual lithium contents, XRD Rietveld refinement, XPS, TG-DSC, and electrochemical measurements are carried out. After secondly treating process, residual lithium contents decrease drastically, and occupancy of Ni in 3a site is much lower and Li/Ni disorder decreases. The discharge capacity is 193.1, 189.7, and 182 mAh g−1 at 0.1 C rate, respectively, for LiNi0.8Mn0.1Co0.1O2-AP, -NT, and -CT electrodes between 3.0 and 4.2 V in pouch cell. The capacity retention has been greatly improved during gradual capacity fading of cycling at 1 C rate. The noticeably improved thermal stability of the samples after being treated can also be observed.








Similar content being viewed by others
References
Armand M, Arascon JM (2008) Building better batteries. Nature 451:652
Manthiram A, Murugan AV, Sarkar A, Muraliganth T (2008) Nanostructured electrode materials for electrochemical energy storage and conversion. Energy Environ Sci 1:621
Goodenough JB, Kim Y (2010) Challenges for rechargeable li batteries. Chem Mater 22:587
Dunn B, Kamath H, Tarascon JM (2011) Electrical energy storage for the grid: a battery of choices. Science 334:928
Zheng HH, Sun Q, Liu G, Song XY (2012) Correlation between dissolution behavior and electrochemical cycling performance for LiNi1/3Co1/3Mn1/3O2-based cells. J Power Sources 207:134
He ZJ, Wang ZX, Huang ZM, Chen H, Li XH, Guo HJ (2015) A novel architecture designed for lithium rich layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 oxides for lithium-ion batteries. J Mater Chem A 3(32):16817
He ZJ, Wang ZX, Chen H, Huang ZM, Li XH, Guo HJ, Wang RH (2015) Electrochemical performance of zirconium doped lithium rich layered Li1.2Mn0.54Ni0.13Co0.13O2 oxide with porous hollow structure. J Power Sources 299:334
Sun ST, Du CQ, Qu DY, Zhang XH, Tang ZY (2015) Li2ZrO3-coated LiNi0.6Co0.2Mn0.2O2 for high-performance cathode material in lithium-ion battery. Ionics 21:2091
Dahn JR, Fuller EW, Obrovac M, von Sacken U (1994) Thermal stability of LixCoO2, LixNiO2 and λ-MnO 2 and consequences for the safety of li-ion cells. Solid State Ionics 69:265
Arai H, Okada S, Ohtsuka H, Ichimura M, Yamaki J (1995) Characterization and cathode performance of Li1−xNi1+xO2 prepared with the excess lithium method. Solid State Ionics 80:261
He ZJ, Ping J, Yi ZJ, Peng C, Shen CS, Liu JS (2017) Optimally designed interface of lithium rich layered oxides for lithium ion battery. J Alloys Compd 708:1038
Matsumoto K, Kuzuo R, Takeya K, Yamanaka A (1999) Effects of CO2 in air on li deintercalation from LiNi1-x-yCoxAlyO2. J Power Sources 81–82:558
Shizuka K, Kiyohara C, Shima K, Takeda Y (2007) Effect of CO2 on layered Li1+zNi1−x−yCoxMyO2 (M = al, Mn) cathode materials for lithium ion batteries. J Power Sources 166:233
Zhang XY, Jiang WJ, Zhu XP, Maugera A, Liu Q, Julien CM (2011) Aging of LiNi1/3Mn1/3Co1/3O2 cathode material upon exposure to H2O. J Power Sources 196:5102
Wooa SW, Myungb ST, Banga H, Kima DW, Sun YK (2009) Improvement of electrochemical and thermal properties of Li[Ni0.8Co0.1Mn0.1]O2 positive electrode materials by multiple metal (Al, Mg) substitution. Electrochim Acta 54:3851
Du R, Bia YJ, Yang WC, Peng Z, Liu M, Liu Y, Wu BM, Yang BC, Ding F, Wang DY (2015) Improved cyclingstability of LiNi0.8Co0.1Mn0.1O2 via Ti substitution with a cut-off potential of 4.5 V. Ceram Int 41:7133
Liang LW, Hu GR, Cao YB, Du K, Peng ZD (2015) Synthesis and characterization of full concentration-gradient LiNi0.7Co0.1Mn0.2O2 cathode material for lithium-ion batteries. J Alloys Compd 635:92
Lee EJ, Noh HJ, Yoon CS, Sun YK (2015) Effect of outer layer thickness on full concentration gradient layered cathode material for lithium-ion batteries. J Power Sources 273:663
Bi YJ, Yang WC, Du R, Zhou JJ, Liu M, Liu Y, Wang DY (2015) Correlation of oxygen non-stoichiometry to the instabilities and electrochemical performance of LiNi0.8Co0.1Mn0.1O2 utilized in lithium ion battery. J Power Sources 283:211
Wu F, Tian J, Su YF, Wang J, Zhang CZ, Bao LY, He T, Li JH, Chen S (2015) Effect of Ni2+ content on lithium/nickel disorder for Ni-Rich cathode materials. ACS Appl Mater Interfaces 7:7702
Li J, Downie LE, Ma L, Qiu WD, Dahn JR (2015) Study of the failure mechanisms of LiNi0.8Co0.1Mn0.1O2 cathode. J Electrochem Soc 162(7):A1401
Wang ZG, Wang ZX, Guo HJ, Peng WJ, Li XH, Wang JX (2014) Enhanced high-voltage electrochemical performance of LiCoO2 coated with ZrOxFy. Mater Lett 123:93
Lee EH, Cho JH, Kim JM, Park JH, Lee SY (2014) Facile surface modification of high-voltage lithium-ion battery cathode materials with electroconductive zinc antimonate colloidal nanoparticles. RSC Adv 4:15630
Kim YS (2013) Encapsulation of LiNi0.5Co0.2Mn0.3O2 with a thin inorganic electrolyte film to reduce gas evolution in the application of lithium ion batteries. Phys Chem 15:6400
Meng K, Wang ZX, Guo HJ, Li XH, Wang D (2016) Improving the cycling performance of LiNi0.8Co0.1Mn0.1O2 by surface coating with Li2TiO3. Electrochim Acta 211:822
Liang LW, Hu GR, Jiang F, Cao YB (2016) Electrochemical behaviours of SiO2-coated LiNi0.8Co0.1Mn0.1O2 cathode materials by a novel modification method. J Alloys Compd 657:570
Li JG, Wang L, Zhang Q, He XM (2009) Synthesis and characterization of LiNi0.6Mn0.4-xCoxO2 as cathode materials for Li-ion batteries. J Power Sources 189:28
Moses AW, Garcia Flores HG, Kim JG, Langell MA (2007) Surface properties of LiCoO2, LiNiO2 and LiNi1-xCoxO2. Appl Surf Sci 253:4782
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21501015) and the Hunan Provincial Natural Science Foundation of China (No. 2016JJ3007).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tang, Z., Zheng, H., Qian, F. et al. Improvement of cycling and thermal stability of LiNi0.8Mn0.1Co0.1O2 cathode material by secondly treating process. Ionics 24, 61–71 (2018). https://doi.org/10.1007/s11581-017-2179-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2179-6