Abstract
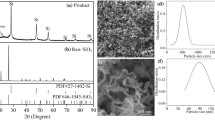
A cost-effective facile approach has been developed to prepare Se nanowires in anodic aluminum oxide (AAO) nanochannels by a mechanical injection method, which enables injection of molten Se into the AAO template to form nanowires during solidification. The as-synthesized Se nanowires display well crystallized as a single phase with a hexagonal structure. In addition, thermal stability and electrochemical properties of Se nanowires were presented and discussed in detail. It was found that the Se nanowires used as a cathode material of Li-Se battery displayed enhanced chemical reaction processes with lithium ions, with a higher storage capacity of 1425.6 mAh g−1 compared with the capacity of bulk Se cathode with 454 mAh g−1 at a current density of 150 mA g−1. The current method of synthesizing nanowires is feasible for other pure elements and their compounds with relatively lower melting points (<650 °C).











Similar content being viewed by others
References
Hu J, Odom TW, Lieber CM (1999) Chemistry and physics in one dimension: synthesis and properties of nanowires and nanotubes. Acc Chem Res 32:435–445
Mieszawska AJ, Slawinski GW, Zamborini FP (2006) Directing the growth of highly aligned gold nanorods through a surface chemical amidation reaction. J Am Chem Soc 128:5622–5623
Whitesides GM, Grzybowski BA (2006) Self-assembly at all scales. Science 295:2418–2421
Pan ZW, Dai ZR, Wang ZL (2001) Nanobelts of semiconducting oxides. Science 291:1947–1949
Duan X, Huang Y, Agarwal R, Lieber CM (2003) Single-nanowire electrically driven lasers. Nature 421:241–245
Zhong Y, Yang M, Zhou X (2015) Structural design for anodes of lithium-ion batteries: emerging horizons from materials to electrodes. Mater Horiz 2:553–566
Chung SW, Yu JY, Health JR (2000) Silicon nanowire devices. Appl Phys Lett 76:2068–2070
Xia Y, Yang P, Sun Y (2003) One-dimensional nanostructures: synthesis, characterization, and applications. Adv Mater 34:353–389
Mayers B, Gates B, Yin YD, Xia YN (2001) Large-scale synthesis of monodisperse nanorods of Se/Te alloys through a homogeneous nucleation and solution growth process. Adv Mater 13:1380–1384
Feng X, Hangarter C, Yoo B (2008) Recent progress in electrodeposition of thermoelectric thin films and nanostructures. Acta Electrochim 53:8103–8117
Chen CC, Fang D, Luo Z (2012) Fabrication and characterization of highly-ordered valve-metal oxide nanotubes and their derivative nanostructures. Rev Nanosci Nanotechnol 1:229–256
Zhang J, Cao Y, Qiang G (2013) Template-assisted nanostructure fabrication by glancing angle deposition: a molecular dynamics study. Nanoscale Res Lett 8:3490–3496
Rambo CR, Recouvreux DOS, Carminatti CA (2008) Template assisted synthesis of porous nanofibrous cellulose membranes for tissue engineering. Mater Sci Eng C 28:549–554
Liu C, Ma D, Ji X (2011) Surfactant assisted synthesis of lamellar nanostructured LiFePO4 at 388 K. Appl Surf Sci 257:4529–4531
Yan C, Xue D, Zou L (2011) A solution-phase approach to the chemical synthesis of ZnO nanostructures via a low-temperature route. J Alloys Compd 453:87–92
Abdullah QN, Yam FK, Hassan JJ (2013) High performance room temperature GaN-nanowires hydrogen gas sensor fabricated by chemical vapor deposition (CVD) technique. Int J Hydrog Energy 38:14085–14101
Battiston AA, Bitter JH (2003) Evolution of Fe species during the synthesis of over-exchanged Fe/ZSM5 obtained by chemical vapor deposition of FeCl3. J Catal 213:251–271
Song Y, Wang Y, Li BB (2013) Interface interaction induced ultra-dense nanoparticles assemblies. Nano 5:6779–6789
Li LC, Fang D, Li GZ, Liu RN, Liu SQ, Xu WL (2016) Mechanism and influence factors of valve-metal oxide nanotube arrays prepared by anodization process. Prog Chem 589-606
Mei X, Kim D, Ruda HE (2002) Molecular-beam epitaxial growth of GaAs and InGaAs/GaAs nanodot arrays using anodic Al2O3 nanohole array template masks. Appl Phys Lett 81:361–363
Musselman KP, Mulholl GJ, Robinson AP (2008) Low-temperature synthesis of large-area, free-standing nanorod arrays on ITO/glass and other conducting substrates. Adv Mater 20:4470–4475
Rajalakshmi M, Arora AK (1999) Optical properties of selenium nanoparticles dispersed in polymer. Solid State Commun 110:75–80
Peng X, Manna L, Yang W (2006) Shape control of CdSe nanocrystals. Nature 404:59–61
Zu L, Norris DJ, Kennedy TA (2006) Impact of ripening on manganese-doped ZnSe nanocrystals. Nano Lett 6:334–340
Gates B, Mayers B, Wu Y (2006) Synthesis and characterization of crystalline Ag2Se nanowires through a template-engaged reaction at room temperature. Adv Funct Mater 12:679–686
Abouimrane A, Dambournet D, Chapman KW, Chupas PJ, Weng W, Amine KA (2012) New class of lithium and sodium rechargeable batteries based on selenium and selenium-sulfur as a positive electrode. J Am Chem Soc 134:4505–4508
Han K, Liu Z, Ye H, Dai F (2014) Flexible self-standing grapheme-Se@CNT composite film as a binder-free cathode for rechargeable li-se batteries. J Power Sources 263:85–89
Luo C, Xu Y, Zhu Y, Liu Y, Zheng S, Liu Y, Langrock A, Wang C (2013) Selenium@mesoporous carbon composite with superior lithium and sodium storage capacity. ACS Nano 7:8003–8010
Liu LL, Hou YY, Wu XW, Xiao SY, Chang Z, Yang YQ, Wu YP (2013) Nanoporous selenium as a cathode material for rechargeable lithium-selenium batteries. Chem Commun 49:11515–11517
Jiang S, Zhang Z, Lai Y, Qu Y, Wang X, Li J (2014) Selenium encapsulated into 3D interconnected hierarchical porous carbon aerogels for lithium-selenium batteries with high rate performance and cycling stability. J Power Sources 267:394–404
Yang CP, Xin S, Yin YX, Ye H, Zhang J, Guo YG (2013) An advanced selenium-carbon cathode for rechargeable lithium-selenium batteries. Angew Chem Int Ed 52:8363–8367
Liu L, Hou Y, Yang Y, Li M, Wang X, Wu Y (2014) A Se/C composite as cathode material for rechargeable lithium batteries with good electrochemical performance. RSC Adv 4:9086–9091
Zhang X, Wang W, Wang A (2014) Improved cycle stability and high security of Li-B alloy anode for lithium-sulfur battery. J Mater Chem a 2:11660–11665
Zhang ZA, Zhang ZY, Zhang K, Yang X, Li Q (2014) Improvement of electrochemical performance of rechargeable lithium-selenium batteries by inserting a free-standing carbon interlayer. RSC Adv 4:15489–15492
Kundu D, Krumeich F, Nesper R (2013) Investigation of nano-fibrous selenium and its polypyrrole and graphene composite as cathode material for rechargeable Li-batteries. J Power Sources 236:112–117
Zhang SY, Zhang J, Wang HY (2004) Synthesis of selenium nanoparticles in the presence of polysaccharides. Mater Lett 58:2590–2594
Jiang ZY, Xie ZX, Xie SY (2003) High purity trigonal selenium nanorods growth via laser ablation under controlled temperature. Chem Phys Lett 368:425–429
Gates B, Mayers B, Grossman A (2002) A Sonochemical approach to the synthesis of crystalline selenium nanowires in solutions and on solid supports. Adv Mater 14:1749–1752
Lu Q, Gao F, Komarneni S (2006) Cellulose-directed growth of selenium nanobelts in solution. Chem Mater 18:159–163
Cao XB, Xie Y, Zhang SY (2014) Ultra-thin trigonal selenium nanoribbons developed from series-wound beads. Adv Mater 16:649–653
Fang D, Li L, Xu W (2016) High capacity lithium ion battery anodes using Sn nanowires encapsulated Al2O3 tubes in carbon matrix. Adv Mater Interfaces 3:243–244
Seah MP (2010) Ultrathin SiO2 on Si. VI. Evaluation of uncertainties in thickness measurement using XPS. Surf Interface Anal 37:300–309
Zhang J, Fan L, Zhu Y (2014) Selenium/interconnected porous hollow carbon bubbles composites as the cathodes of Li-Se batteries with high performance. Nano 6:12952–12957
Jiang X, Kemal L, Yu A (2007) Silver-induced growth of selenium nanowires in aqueous solution. Mater Lett 61:2584–2588
Gates B, Yin Y, Xia Y (2001) ChemInform abstract: a solution-phase approach to the synthesis of uniform nanowires of crystalline selenium with lateral dimensions in the range of 10–30 nm. Cheminf 32:12582–12583
Li XM, Li Y, Li SQ (2005) Single crystalline trigonal selenium nanotubes and nanowires synthesized by sonochemical process. Cryst Growth Des 5:911–916
Song JM, Zhu AJH, Yu SH (2006) Crystallization and shape evolution of single crystalline selenium nanorods at liquid-liquid Interface: from monodisperse amorphous Se nanospheres toward Se nanorods. J Phys Chem B 110:23790–23795
Luo Z (2016) A practical guide to transmission electron microscopy, volume I: fundamentals. Momentum Press, New York, p 6
Ray C, Dutta S, Sarkar S (2013) A facile synthesis of 1D nano structured selenium and Au decorated nano selenium: catalysts for the clock reaction. RSC Adv 3:24313–24320
Ge XL, Wang XD (2009) Calculations of freezing point depression, boiling point elevation, vapor pressure and enthalpies of vaporization of electrolyte solutions by a modified three-characteristic parameter correlation model. J Solut Chem 38:1097–1117
Wautelet M, Duvivier D (2007) The characteristic dimensions of the nanoworld. Eur J Phys 28:953–959
Roduner E (2006) Size matters: why nanomaterials are different. Chem Soc rev 35:583–592
Fernando D, Nigro TAE, Dyer ID (2016) Synthesis and catalytic activity of the metastable phase of gold phosphide. J Solid State Chem 242:182–192
Cui Y, Abouimrane A, Lu J, Bolin T (2013) (De)lithiation mechanism of Li/SeSx (x = 0–7) batteries determined by in situ synchrotron X-ray diffraction and X-ray absorption spectroscopy. J Am Chem Soc 135:8047–8056
Bao W, Zhang Z, Zhou C (2014) Multi-walled carbon nanotubes@mesoporous carbon hybrid nanocomposites from carbonized multi-walled carbon nanotubes@metal-organic framework for lithium sulfur battery. J Power Sources 248:570–576
Lai Y, Yang F, Zhang Z (2014) Encapsulation of selenium in porous hollow carbon spheres for advanced lithium-selenium batteries. RSC Adv 4:39312–39315
Abouimrane A, Dambournet D, Chapman KW (2012) A new class of lithium and sodium rechargeable batteries based on selenium and selenium-sulfur as a positive electrode. J Am Chem Soc 134:4505–4508
Han K, Liu Z, Shen J (2014) Resonant bonding in crystalline phase-change materials. Adv Funct Mater 25:455–463
Zhou X, Gao P, Sun S (2015) Crystalline and crystalline/amorphous selenium nanowires and their different (de)lithiation mechanisms. Chem Mater 27:6730–6736
Zhu J, Hu G, Zhang J (2016) Preparation of Sn-Cu-graphene nanocomposites with superior reversible lithium ion storage. Mater Lett 185:565–568
Yi TF, Mei J, Zhu YR (2015) Li5Cr7Ti6O25 as a novel negative electrode material for lithium-ion batteries. Chem Commun 51:14050–14053
Tan Z, Sun Z, Wang H (2013) Fabrication of porous Sn-C composites with high initial coulomb efficiency and good cyclic performance for lithium ion batteries. J Mater Chem A 1:9462–9468
Chen T, Liu Y, Pan L (2014) Electrospun carbon nanofibers as anode materials for sodium ion batteries with excellent cycle performance. J Mater Chem A 2:4117–4121
Ye H, Yin YX, Zhang SF (2014) Advanced Se-C nanocomposites: a bifunctional electrode material for both Li-Se and Li-ion batteries. J Mater Chem A 2:13293–13298
Cheon SE, Ko KS, Cho JH, Kim SW, Chin EY, Kim HT (2003) Rechargeable lithium sulfur battery I. Structural change of sulfur cathode during discharge and charge. J Electrochem Soc 150:A796–A799
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Nos. 51201117, 51104121), the Major State Basic Research Development Program (973 Program) (No. 2012CB722701), the Natural Science Foundation of Hubei Province (No. MCF20140123), the Scientific Research Fund of Wuhan Textile University, and the Scholarship Award for Excellent Doctoral Student granted by Ministry of Education of China (No. 1343-71134001002).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Table S1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Wang, C., Hu, Q., Wei, Y. et al. Facile fabrication of selenium (Se) nanowires for enhanced lithium storage in Li-Se battery. Ionics 23, 3571–3579 (2017). https://doi.org/10.1007/s11581-017-2164-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2164-0