Abstract
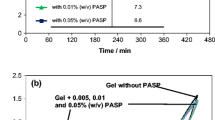
In this study, a novel polysiloxane-based gel agent (PSGA) was synthesized by using poly(dimethylsiloxane-co-alkylmethylsiloxane) and used as a gel agent for gel–valve-regulated lead–acid batteries. A PSGA was characterized by Fourier transform infrared, zeta meter, scanning electron microscopy, and energy-dispersive X-ray analysis. The electrochemical characterization of the gel system for the optimization of some parameters, such as concentration of gel agent, stirring rate, and agitation time, was conducted by cyclic voltammetric and electrochemical impedance spectroscopic analyses. The optimum concentration of the gel agent was determined as 6 wt% of the PSGA to form the best gel structure. The mechanical parameters related to the formation of a suitable gel structure were also investigated. The optimum stirring rate and agitation time were determined as 500 rpm and 2.5 h, respectively. The charge–discharge tests were applied to the gel system, and the highest capacity was determined in the PSGA-based gel system as 10 mAh at the end of the 100th cycle. The capacity of non-gelled system was the lowest.
















Similar content being viewed by others
References
John D (1997) An account of the development of the first valve-regulated lead/acid cell. J Power Sources 64:153–156
Wagner R (2005) High-power lead–acid batteries for different applications. J Power Sources 144:494–504
Chang Y, Mao X, Zhao Y, Feng S, Chen H, Finlow D (2009) Lead-acid battery use in the development of renewable energy systems in China. J Power Sources 191:176–183
Soria ML, Herńandez JC, Valenciano J, Śanchez A, Trinidad F (2005) New developments on valve-regulated lead–acid batteries for advanced automotive electrical systems. J Power Sources 144:473–485
Misra SS (2007) Advances in VRLA battery technology for telecommunications. J Power Sources 168:40–48
Sarrias-Mena R, Fernández-Ramírez LM, García-Vázquez CA, Jurado F (2014) Improving grid integration of wind turbines by using secondary batteries. Renew Sust Energ Rev 34:194–207
Lambert DWH, Greenwood PHJ, Reed MC (2002) Advances in gelled-electrolyte technology for valve-regulated lead-acid batteries. J Power Sources 107:173–179
Prengaman RD (2005) Improvements to active material for VRLA batteries. J Power Sources 144:426–437
An SY, Jeong ED, Won MS, Shim YB (2008) The optimization of gel electrolytes on performance of valve regulated lead acid batteries. Bull Kor Chem Soc 29:998–1002
Chen MQ, Chen HY, Shu D, Li AJ, Finlow DE (2008) Effects of preparation condition and particle size distribution on fumed silica gel valve-regulated lead–acid batteries performance. J Power Sources 181:161–171
Pan K, Shi G, Li A, Li H, Zhao R, Wang F, Zhang W, Chen Q, Chen H, Xiong Z, Finlow D (2012) The performance of a silica-based mixed gel electrolyte in lead acid batteries. J Power Sources 209:262–268
Tantichanakul T, Chailapakul O, Tantaviche N (2013) Influence of fumed silica and additives on the gel formation and performance of gel valve-regulated lead-acid batteries. J Ind Eng Chem 19:2085–2091
Gençten M, Dönmez KB, Şahin Y, Pekmez K, Suvacı E (2014) Voltammetric and electrochemical impedimetric behavior of silica-based gel electrolyte for valve-regulated lead-acid battery. J Solid State Electrochem 18:2569–2479
Chen M, Guo W, Zhang M, Cheng F, Liu P, Cai Z, Zhang Y (2015) Effect of polyols on the electrochemical behavior of gel valve-regulated lead-acid batteries. Electrochim Acta 164:243–251
Vinod MP, Vijayamohanan K, Joshi SN (1998) Effect of silicate and phosphate additives on the kinetics of the oxygen evolution reaction in valve-regulated lead/acid batteries. J Power Sources 70:103–105
Hernandez JC, Soria ML, Gonzalez M, Garcia-Quismondo E, Munoz A, Trinidad F (2006) Studies on electrolyte formulations to improve life of lead acid batteries working under partial state of charge conditions. J Power Sources 162:851–863
Tang Z, Wang J, Mao X, Shao H, Chen Q, Xu Z, Zhang J (2007a) Investigation and application of polysiloxane-based gel electrolyte in valve-regulated lead-acid battery. J Power Sources 168:49–57
Tang Z, Wang J, Mao X, Chen Q, Shen C, Zhang J (2007b) Application of a novel gelled-electrolyte in valve-regulated lead-acid batteries with tubular positive plates. J Appl Electrochem 37:1163–1169
Hamenoja E, Laitinen T, Sundholm G, Yli-Pentti A (1989) The growth of oxide layers on lead and its alloys at a constant potential in the PbO2 potential region at different temperatures. Electrochim Acta 34:233–241
Lee IJ, Song GS, Lee WS, Suh DH (2003) A new class of solid polymer electrolyte: synthesis and ionic conductivity of novel polysiloxane containing allyl cyanide groups. J Power Sources 114:320–329
Kang Y, Lee J, Suh DH, Lee C (2005) A new polysiloxane based cross-linker for solid polymer electrolyte. J Power Sources 146:391–396
Nakahara H, Yoon S, Nutt S (2006) Effect of an additive to polysiloxane-based electrolyte on passive film formation on a graphite electrode. J Power Sources 158:600–607
Raghavan SR, Walls HJ, Khan SA (2000) Rheology of silica dispersions in organic liquids: new evidence for solvation forces dictated by hydrogen bonding. Langmuir 16:7920–7930
Vinod MP, Vijayamohanan K (2000) Effect of gelling on the impedance parameters of Pb/PbSO4 electrode in maintenance-free lead-acid batteries. J Power Sources 89:88–92
Wu L, Chen HY, Jiang X (2002) Effect of silica soot on behaviour of negative electrode in lead–acid batteries. J Power Sources 107:162–166
Gençten M, Gürsu H, Şahin Y (2016) Electrochemical investigation of the effects of V(V) and sulfuric acid concentrations on positive electrolyte for vanadium redox flow battery. Int J Hydrogen Energ 41:9868–9875
Kawaguchi M (1996) Molecular weight dependence of structures and rheological properties for fumed silica suspensions in polystyrene solutions. Langmuir 12:6179–6183
Sun X, Zhao J (2016) Concentration optimization of fumed silica as gelator in lead-acid batteries. Electrochemistry 84:578–584
Guo Y, Niu L, Zhang S, Chen S (2000) The electrochemical behavior of PbSO4 with different structures on Pb. J Power Sources 85:38–43
Lindbergh G (1997) Experimental determination of the effective electrolyte conductivity in porous lead electrodes in the lead-acid battery. Electrochim Acta 42:1239–1246
Calabek M, Mick K, Křivaǩ P, Baca P (2006) Significance of carbon additive in negative lead-acid battery electrodes. J Power Sources 158:864–867
Acknowledgments
This work was supported by the SAN-TEZ program (No. 00897.STZ.2011-1) of the Ministry of Science, Industry and Technology, Republic of Turkey; Anadolu University; and Ericsson Turkey. Y. Şahin thanks Prof. Dr. Kadir Pekmez, Prof. Dr. Ender Suvacı, and Oktay Uysal for their supports to this study. M. Gençten thanks the TUBİTAK-BİDEB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dönmez, K.B., Gençten, M. & Şahin, Y. A novel polysiloxane-based polymer as a gel agent for gel–VRLA batteries. Ionics 23, 2077–2089 (2017). https://doi.org/10.1007/s11581-017-2040-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2040-y