Abstract
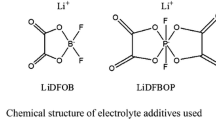
Manganese oxide-based cathodes are one of the most promising lithium-ion battery (LIB) cathode materials due to their cost-effectiveness, high discharge voltage plateau (above 4.0 V vs. Li/Li+), superior rate capability, and environmental benignity. However, these batteries using conventional LiPF6-based electrolytes suffer from Mn dissolution and poor cyclic capability at elevated temperature. In this paper, the ionic liquid (IL)-based electrolytes, consisting of 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfon)imidate (PYR1,4-TFSI), propylene carbonate (PC), lithium bis(trifluoromethanesulfon)imide (LiTFSI), and lithium oxalyldifluoroborate (LiDFOB) additive, were explored for improving the high temperature performance of the LiMn2O4 batteries. It was demonstrated that LiTFSI-ILs/PC electrolyte associated with LiDFOB addition possessed less Mn dissolution and Al corrosion at the elevated temperature in LiMn2O4/Li batteries. Cyclic voltammetry and electrochemical impedance spectroscopy implied that this kind of electrolyte also contributed to the formation of a highly stable solid electrolyte interface (SEI), which was in accordance with the polarization measurement and the Li deposition morphology of the symmetric lithium metal cell, thus beneficial for improving the cycling performance of the LiMn2O4 batteries at the elevated temperature. Cyclic voltammetry and electrochemical impedance spectroscopy implied that the cells using this kind of electrolyte exhibited better interfacial stability, which was further verified by the polarization measurement and the Li deposition morphology of the symmetric lithium metal cell, thus beneficial for improving the cycling performance of the LiMn2O4 batteries at the elevated temperature. These unique characteristics would endow this kind of electrolyte a very promising candidate for the manganese oxide-based batteries.







Similar content being viewed by others
References
Goodenough JB, Park KS (2013) J Am Chem Soc 135:1167
Ritchie A, Howard W (2006) J Power Sources 162:809
Dunn B, Kamath H, Tarascon J-M (2011) Science 334:928
Thackeray MM, Wolverton C, Isaacs ED (2012) Energy Environ. Sci. 5:7854
Bresser D, Passerini S, Scrosati B (2013) Chem Commun 49:10545
Bruce PG, Freunberger SA, Hardwick LJ, Tarascon J-M (2012) Nat Mater 11:19
A. M, Tarascon J-M (2001) Nature 414:9
Scrosati B, Garche J (2010) J Power Sources 195:2419
Scrosati B, Hassoun J, Sun Y-K (2011) Energy Environ. Sci. 4:3287
Sun Y-K, Lee Y-S, Yoshio M, Amine K (2002) Electrochem Solid-State Lett 5:A99
Ammundsen B, Paulsen J (2001) Adv Mater 13:943
Desilvestro J, Haas O (1990) J Electrochem Soc 137:5C
Qin B, Liu Z, Ding G, Duan Y, Zhang C, Cui G (2014) Electrochim Acta 141:167
Xu G, Liu Z, Zhang C, Cui G, Chen L (2015) J Mater Chem A 3:4092
Botte GG, White RE, Zhang Z (2001) J Power Sources 97–98:570
Kawamura T, Okada S, Yamaki J-i (2006) J Power Sources 156:547
Sun Y-K, Myung S-T, Park B-C, Prakash J, Belharouak I, Amine K (2009) Nat Mater 8:320
Hu P, Duan Y, Hu D, Qin B, Zhang J, Wang Q, Liu Z, Cui G, Chen L (2015) ACS Appl Mat Interfaces 7:4720
Shin J-H, Henderson WA, Passerini S (2003) Electrochem Commun 5:1016
Gebresilassie Eshetu G, Armand M, Scrosati B, Passerini S (2014) Angew Chem Int Ed 53:13342
Kühnel RS, Böckenfeld N, Passerini S, Winter M, Balducci A (2011) Electrochim Acta 56:4092
Kühnel R-S, Balducci A (2014) J Phys Chem C 118:5742
Kühnel R-S, Lübke M, Winter M, Passerini S, Balducci A (2012) J Power Sources 214:178
Gao X-W, Feng C-Q, Chou S-L, Wang J-Z, Sun J-Z, Forsyth M, MacFarlane DR, Liu H-K (2013) Electrochim Acta 101:151
Swiderska-Mocek A (2014) J Solid State Electrochem 18:1077
Wongittharom N, Lee T-C, Hung IM, Lee S-W, Wang Y-C, Chang J-K (2014) J Mater Chem A 2:3613
Hofmann A, Werth F, Howeling A, Hanemann T (2015) ECS Electrochem Lett 4:A141
Xu K (2004) Chem Rev 104:116
Qin B, Liu Z, Zheng J, Hu P, Ding G, Zhang C, Zhao J, Kong D, Cui G (2015) J Mater Chem A 3:7773
Menne S, Kühnel RS, Balducci A (2013) Electrochim Acta 90:641
Park M, Zhang X, Chung M, Less GB, Sastry AM (2010) J Power Sources 195:7904
Park K, Yu S, Lee C, Lee H (2015) J Power Sources 296:197
Miao R, Yang J, Feng X, Jia H, Wang J, Nuli Y (2014) J Power Sources 271:291
Xu W, Wang J, Ding F, Chen X, Nasybulin E, Zhang Y, Zhang J-G (2014) Energy Environ Sci 7:513
Lu Y, Xu S, Shu J, Aladat WIA, Archer LA (2015) Electrochem Commun 51:23
Leroy S, Martinez H, Dedryvere R, Lemordant D, Gonbeau D (2007) App Surf Sci 253:4895
Choudhury S, Archer LA (2016) Adv Electron Mater 2:1500246
Chen Z, Qin Y, Liu J, Amine K (2009) Electrochem Solid-State Lett 12:A69
Xu M, Zhou L, Hao L, Xing L, Li W, Lucht BL (2011) J Power Sources 196:6794
Xia Y, Zhou Y, Yoshio M (1997) J Electrochem Soc 144:2593
Lu D, Li W, Zuo X, Yuan Z, Huang Q (2007) J Phys Chem C 111:12067
Levi MD, Aurbach D (1997) J Phys Chem B 101:4630
Acknowledgements
This work was supported by the National Program on the National High Technology Research and Development Program of China (863 program, No. 2013AA050905), Key Project of Natural Science Foundation of Shandong Province (ZR2015QZ01), “135” Projects Fund of CAS-QIBEBT Director Innovation Foundation, and Qingdao Institute of Bioenergy and Bioprocess Technology Director Technology Foundation.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Bingsheng Qin and Shu Zhang contributed equally to this work.
Electronic supplementary material
.
ESM 1
(DOCX 241 kb)
Rights and permissions
About this article
Cite this article
Qin, B., Zhang, S., Hu, Z. et al. Ionic liquid-based electrolyte with dual-functional LiDFOB additive toward high-performance LiMn2O4 batteries. Ionics 23, 1399–1406 (2017). https://doi.org/10.1007/s11581-016-1966-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1966-9