Abstract
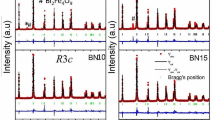
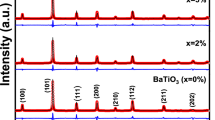
In this work, we research two series of Mn-substituted bismuth molybdates: Bi26-2xMn2xMo10O69-d and Bi26Mo10-2yMn2yO69-d. The synthesis of powder samples is performed by the conventional solid state technology. Samples are characterized by X-ray diffraction, scanning electron microscopy, and chemical analysis methods, and it is shown that single phase Bi26-2xMn2xMo10O69-d and Bi26Mo10-2yMn2yO69-d complex oxides form up to x = 0.8 and y = 0. We use densitometry, grain size measurements and scanning electron microscopy to study the morphology of ceramic pellets and powders. This issue reveals formation of dense ceramic samples with low porosity (≤3%). High-temperature X-ray diffraction is used to define small deviation of unit cell parameters from their linear dependence on temperature. Measurement of electrical conductivity is made using a.c. impedance spectroscopy method. We observe the decrease of electrical conductivity in Bi26-2xMn2xMo10O69-d series depending on dopant concentration.







Similar content being viewed by others
References
Vedrine JC, Coudurier G, Forissier M, Volta JC (1985) Catalytic properties of metallic oxides in partial oxidation reactions. Mat Chem&Phys 13:365–378. doi:10.1016/0254-0584(85)90065-3
Zhao X, Xu T, Yao W, Zhu Y (2009) Synthesis and photoelectrochemical properties of thin bismuth molybdates film with various crystal phases. Thin Solid Films 517:5813–5818. doi:10.1016/j.tsf.2009.02.135
Boivin J-C (2001) Structural and electrochemical features of fast oxide ion conductors. Int J Inorg Mat 3:1261–1266. doi:10.1016/s1466-6049(01)00118-0
Sharma N, Macquart RB, Christensen M, Avdeev M, Chen YS, Ling CD (2009) Structure and crystal chemistry of fluorite-related Bi38Mo7O78 from single crystal X-ray diffraction and ab initio calculations. J Solid State Chem 182:1312–1318. doi:10.1016/j.jssc.2009.02.030
Galy J, Enjalbert R, Rozier P, Millet P (2003) Lone pair stereoactivity versus anionic conductivity. Columnar structures in the Bi2O3–MoO3 system. Solid State Sci 5:165–174. doi:10.1016/s1293-2558(02)00090-0
Thompson JG, Schmid S, Withers RL, Rae AD (1992) Comparison of the crystal structures of γ-Bi2MoO6 and Bi2WO6. J Solid State Chem 101:309–321. doi:10.1016/0022-4596(92)90186-Y
Buttrey DJ, Vogt T, Yap GPA, Rheingold AL (1997) The structure of Bi26Mo10O69. Mater Res Bull 32:947–962. doi:10.1016/s0025-5408(97)00063-9
Muktha B, Aarthi T, Madras G, Guru Row TN (2006) Substitution effect on the photocatalytic degradation by the series AxBi26-xMo10O68-0.5y (A=Ba, y= 0; A=Bi, La, y= 2): a kinetic study. J Phys Chem B 110:10280–10286. doi:10.1021/jp060945o
Vannier RN, Mairesse G, Abraham F, Nowogorski G (1996) Bi26Mo10OδSolid solution type in the Bi2O3–MoO3–V2O5 ternary diagram. J Solid State Chem 122:394–406. doi:10.1006/jssc.1996.0133
Ling CD, Miiller W, Johnson MR, Richard D, Rols S, Madge J, Evans IR (2012) Local structure, dynamics, and the mechanisms of oxide ionic conduction in Bi26Mo10O69. Chem Mater 24:4607–4614. doi:10.1021/cm303202r
Bastide B, Enjalbert R, Salles P, Galy J (2003) Ionic conductivity of the oxide family Bi[Bi12O14][(Mo,M)O4]5 with M = Li, Mg, Al, Si, Ge and V. Solid State Ionics 158:351–358. doi:10.1016/s0167-2738(02)00910-4
Vannier RN, Danze S, Nowogrocki G, Huve M, Mairesse G (2000) A new class of mono-dimensional bismuth-based oxide anion conductors with a structure based on [Bi12O14]∞ columns. Solid State Ionics 136–137:51–59. doi:10.1016/S0167-2738(00)00351-9
Mikhailovskaya ZA, Buyanova ES, Petrova SA, Morozova MV, Zhukovskiy VM, Zakharov RG, Tarakina NV, Berger IF (2013) Cobalt-doped Bi26Mo10O69 : crystal structure and conductivity. J Solid State Chem 204:9–15. doi:10.1016/j.jssc.2013.05.006
Begue P, Rojo JM, Iglesias E, Castro A (2002) Different [Bi12O14]n columnar structural types in the Bi-Mo-Cr-O system: synthesis, structure, and electrical properties of the solid solution Bi26Mo10-xCrxO69. J Solid State Chem 166:7–14. doi:10.1006/jssc.2002.9543
Enjalbert R, Hasselmann G, GalyJ (1997) A new mixed oxide with (Bi12O14)n columns: PbBi12Mo5O34. ActaCrystallogrC 53:269–272. doi:10.1107/s0108270196013698
Galy J, Salles P, Rozier P, Castro A (2006) Anionic conductors Ln2/3[Bi12O14](MoO4)5 with Ln=La, Nd, Gd, Ho, Yb. Synthesis–spark plasma sintering–structure–electric properties. Solid State Ionics 177:2897–2902. doi:10.1016/j.ssi.2006.07.059
Muktha B, Guru Row TN (2007) Crystal structure and ionic conductivity of the series A2Bi24Mo8X2O68 (A=Ca, Sr and Ba and X=Cr, W). StructChem 18:195–202. doi:10.1007/s11224-006-9093-2
Castro A, Enjalbert R, Baules P, Galy J (1998) Synthesis and structural evolution of the solid solution Bi(Bi12−xTexO14)Mo4−xV1+xO20(0≤x<2.5). J Solid State Chem 139:185–193. doi:10.1006/jssc.1998.7831
Mikhailovskaya ZA, Buyanova ES, Petrova SA, Zhukovskii VM (2013) Oxygen–ionic conductors based on substituted bismuth molybdates with column-type structural fragments. Russ J Electrochem 49(7):658–664. doi:10.1134/S1023193513070112
Mikhaylovskaya ZA, Morozova MV, Buyanova ES, Petrova SA, Abrahams I (2014) Iron-doped Bi26Mo10O69 bismuth molybdate: synthesis, properties and structure. In: Abstract Book. of the 11th international symposium on systems with fast ionic transport, Gdańsk University of Technology, Gdańsk-Sobieszewo, Poland, 25–29 June 2014, p.78.
DIFFRACPlus: EvaBruker AXS GmbH, Ostliche. Rheinbruckenstraße 50, D-76187, Karlsruhe, Germany. 2008
Powder Diffraction File PDF4+ ICDD Release 2014
Laugier J, Bochu B (2003) LMGP-Suite of programs for the interpretation of X- ray Experiments. ENSP/Lab Materiauxgenie Phys, Grenoble
DIFFRACPlus (2008) TOPAS Bruker AXSGmbH, Ostliche. Rheinbruckenstraße 50, D-76187, Karlsruhe, Germany
Mikhaylovskaya ZA, Buyanova ES, Morozova MV, Petrova SA, Zakharov RG, Nikolaenko IV, Abrahams I (2015) Bi13-xMexMo5O34±δ (Me = Mg, Ca, Sr, Ba) solid solutions: synthesis and properties. Ionics 21:2259–2268. doi:10.1007/s11581-015-1421-3
Irvine JTS, Sinclair DC, West AR (1990) Electroceramics: characterization by impedance spectroscopy. Adv Mater 2:132–138. doi:10.1002/adma.19900020304
Acknowledgements
This work was financially supported by the Russian Foundation for Basic Research (project No 16-33-60026) and Ministry of Education and Science (grant of President of Russia № МК-7979.2016.3). XRPD data were obtained using the equipment of the Centre for Shared Use ‘Ural-M’
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mikhaylovskaya, Z.A., Buyanova, E.S., Morozova, M. et al. Mn-doped Bi26Mo10O69-d: synthesis and characterization. Ionics 23, 1107–1114 (2017). https://doi.org/10.1007/s11581-016-1917-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1917-5