Abstract
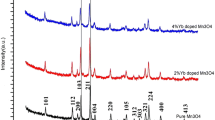
Aluminum doped MnO2 nanoparticles were synthesized by a simple liquid-phase process using potassium permanganate as oxidation agent, glycol as reducing agent. Specific capacitance of the optimal sample electrode can reach 290 F g−1 after 10 cycles. The electrode also exhibits excellent cycle stability, retaining 86.6 % after 1,000 cycles. The infrared absorption bands of aluminum doped manganese oxide shift to high wave number for the reason that aluminum ion has smaller nuclear charge. The doping of aluminum strengthens the Mn–O bond and decreases the aggregation degree, thus the electrochemical properties are enhanced.











Similar content being viewed by others

References
Toupin M, Brousse T, Belanger D (2004) Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem Mater 16:3184–3190
Chang JK, Tsai WT (2003) Material characterization and electrochemical performance of hydrous manganese oxide electrodes for use in electrochemical pseudocapacitors. J Electrochem Soc 150:A1333–A1338
Chun SE, Pyun SI, Lee GJ (2006) A study on mechanism of charging/discharging at amorphous manganese oxide electrode in 0.1m Na2SO4 solution. Electrochim Acta 51:6479–6486
Li L, Qin ZY, Wang LF, Liu HJ, Zhu MF (2010) Anchoring alpha-manganese oxide nanocrystallites on multi-walled carbon nanotubes as electrode materials for supercapacitor. J Nanopart Res 12:2349–2353
Subramanian V, Zhu H, Wei B (2008) Alcohol-assisted room temperature synthesis of different nanostructured manganese oxides and their pseudocapacitance properties in neutral electrolyte. Chem Phys Lett 453:242–249
Jayalakshmi M, Balasubramanian K (2008) Simple capacitors to supercapacitors-an overview. Int J Electrochem Sci 3:1196–1217
Raymundo-Pinero E, Khomenko V, Frackowiak E, Beguin F (2005) Performance of manganese oxide/CNTs composites as electrode materials for electrochemical capacitors. J Electrochem Soc 152:A229–A235
Ye C, Lin ZM, Hui SZ (2005) Electrochemical and capacitance properties of rod-shaped MnO2 for supercapacitor batteries, fuel cells, and energy conversion. J Electrochem Soc 152:A1272–A1278
Hu CC, Tsou TW (2002) Ideal capacitive behavior of hydrous manganese oxide prepared by anodic deposition. Electrochem Commun 4:105–109
Lang XY, Hirata A, Fujita T, Chen MW (2011) Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat Nanotechnol 6:232–236
Yan J, Khoo E, Sumboja A, Lee PS (2010) Facile coating of manganese oxide on tin oxide nanowires with high-performance capacitive behavior. ACS Nano 4:4247–4255
Song MK, Cheng S, Chen HY (2012) Anomalous pseudocapacitive behavior of a nanostructured, mixed-valent manganese oxide film for electrical energy storage. Nano Lett 12:4416–4416
Babakhani B, Ivey DG (2010) Anodic deposition of manganese oxide electrodes with rod-like structures for application as electrochemical capacitors. J Power Sources 195:2110–2117
Toupin M, Brousse T, Bélanger D (2002) Influence of microstructure on the charge storage properties of chemically synthesized manganese dioxide. Chem Mater 14:3946–3952
Lee HY, Kim SW, Lee HY (2001) Expansion of active site area and improvement of kinetic reversibility in electrochemical pseudocapacitor electrode. Electrochem Solid State Lett 4:A19–A22
Subramanian V, Zhu HW, Wei BQ (2006) Nanostructured MnO2: hydrothermal synthesis and electrochemical properties as a supercapacitor electrode material. J Power Sources 159:361–364
Wang YT, Lu AH, Zhang HL, Li WC (2011) Synthesis of nanostructured mesoporous manganese oxides with three-dimensional frameworks and their application in supercapacitors. J Phys Chem C 115:5413–5421
Xiao LF, Zhao YQ, Yang YY (2008) Enhanced electrochemical stability of al-doped LiMn2O4 synthesized by a polymer-pyrolysis method. Electrochim Acta 54:545–550
Taniguchi I, Song D, Wakihara M (2002) Electrochemical properties of LiM1/6Mn11/6O4 (M=Mn, Co, Al, Ni) as cathode materials for Li-ion batteries prepared by ultrasonic spray pyrolysis method. J Power Sources 109:333–339
Eftehari A (2004) Aluminum oxide as a multi-function agent for improving battery performance of LiMn2O4 cathode. Solid State Ionics 167:237–242
Dokko K, Hoshina K, Nakano H, Kanamura K (2007) Preparation of LiMn2O4 thin-film electrode on Li1+xAlxTi2−x(PO4)3 nasicon-type solid electrolyte. J Power Sources 174:1100–1103
Kim KW, Lee SW, Han KS et al (2003) Characterization of al-doped spinel LiMn2O4 thin film cathode electrodes prepared by liquid source misted chemical deposition (LSMCD) technique. Electrochim Acta 48:4223–4231
Yu P, Zhang X, Wang DL, Wang L, Ma YW (2009) Shape-controlled synthesis of 3D hierarchical MnO2 nanostructures for electrochemical supercapacitors. Cryst Growth Des 9:528–533
Li Y, Xie HQ (2010) Mechanochemical-synthesized al-doped manganese dioxides for electrochemical supercapacitors. Ionics 16:21–25
Zhou Q, Li X, Li YG, Tian BZ, Zhao DY, Jiang ZY (2006) Synthesis and electrochemical properties of semicrystalline gyroidal mesoporous MnO2. Chi J Chem 24:835–839
Lee JA, Newnham CE, Tye FL (1973) Energetics of water desorption from a γ-manganese dioxide. J Colloid Interface SCi 42:372–380
Ragupathy P, Park DH, Campet G, Vasan HN, Hwang SJ, Choy JH (2009) Remarkable capacity retention of nanostructured manganese oxide upon cycling as an electrode material for supercapacitor. J Phys Chem C 113:6303–6309
Yuan A, Zhang Q (2006) A novel hybrid manganese dioxide/activatedcarbon supercapacitor using lithium hydroxide electrolyte. Electrochem Commun 8:1173–1178
Zheng JP, Cygan PJ, Row TR (1995) Hydrous ruthenium oxide as an electrode material for electrochemical capacitors. J Electrochem Soc 142:2699–2703
Ananth MV, Pethkar S, Dakshinamurthi K (1998) Distortion of MnO6 octahedra and electrochemical activity of nstutite-based MnO2 polymorphs for alkaline electrolytes—an FTIR study. J Power Sources 75:278–282
Iwata E, Takahashi K, Maeda T, Mouri T (1999) Capacity failure on cycling or storage of Lithium-ion batteries with Li–Mn–O ternary phases having spinel-framework structure and its possible solution. J Power Sources 81–82:430–433
Fan ZJ, Yan J, Wei T (2011) Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv Funct Mater 21:2366–2375
Kanoh H, Tang W, Makita Y, Ooi K (1997) Electrochemical intercalation of alkali-metal ions into birnessite-type manganese oxide in aqueous-solution. Langmuir 13:6845–6849
Athouël L, Moser F (2008) Variation of the MnO2 birnessite structure upon charge/discharge in an electrochemical supercapacitor electrode in aqueous Na2SO4 electrolyte. J Phys Chem C 112:7270–7277
Qu QT, Li L, Tian S, Guo WL (2010) A cheap asymmetric supercapacitor with high energy at high power: activated carbon//K0.27MnO2 · 0.6H2O. J power Sources 195:2789–2794
Qu QT, Wang B, Yang LC, Shi Y, Tian S, Wu YP (2008) Study on electrochemical performance of activated carbon in aqueous Li2SO4, Na2SO4 and K2SO4 electrolytes. Electrochem Commun 10:1652–1655
Acknowledgments
We are grateful for the financial support from the Natural Science Foundation of Hebei Province (B2012203069) and support from education department of Hebei province on natural science research key projects for institution of higher learning (ZH2011228).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, G., Shao, G., Wang, L. et al. Enhanced electrochemical properties of Al-doped bulk manganese oxides synthesized by a facile liquid-phase method. Ionics 20, 1367–1375 (2014). https://doi.org/10.1007/s11581-014-1104-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1104-5