Abstract
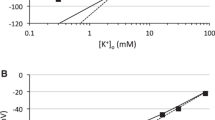
Astrocytes play a critical role in CNS metabolism, regulation of volume and ion homeostasis of the interstitial space. Of special relevance is their clearance of K+ that is released by active neurons into the extracellular space. Mathematical analysis of a modified Nernst equation for the electrochemical equilibrium of neuronal plasma membranes, suggests that K+ uptake by glial cells is not only relevant during neuronal activity but also has a non-neglectable impact on the basic electrical membrane properties, specifically the resting membrane potential, of neurons and might be clinically valuable as a factor in the genetics and epigenetics of the epilepsy and tuberous sclerosis complex.

Similar content being viewed by others
Notes
A constant of proportionality that relates the electric field in a material to the electric displacement in that material. It characterizes the tendency of the atomic charge in an insulating material to distort in the presence of an electric field.
References
Ballanyi K, Grafe P, ten Bruggencate G (1987) Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J Physiol 382:159–174
Barcilon V, Chen D-P, Eisenberg RS (1992) Ion flow through narrow membrane channels: part II. SIAM J Appl Math 52:1405–1425
Barcilon V, Chen D-P, Eisenberg RS, Jerome JW (1997) Qualitative properties of steady-state Poisson-Nernst-Planck systems: perturbation and simulation study. SIAM J Appl Math 57:631–648
Chen KC, Nicholson C (2000) Spatial buffering of potassium ions in brain extracellular space. Biophys J 78(6):2776–2797
Clay JR (2005) Axonal excitability revisited. Prog Biophys Mol Biol 88(1):59–90
Cressman JR Jr, Ullah G, Ziburkus J, Schiff SJ, Barreto E (2009) The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states: I. Single neuron dynamics. J Comput Neurosci 26(2):159–170
D’Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D (1999) Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci 19(18):8152–8162
David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D, Friedman A (2009) Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis?. J Neurosci 29(34):10588–10599
Deitmer JW, Rose CR (2010) Ion changes and signalling in perisynaptic glia. Brain Res Rev 63(1-2):113–129
Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD (2007) Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27(42):11354–11365
Gardner-Medwin AR (1983) Analysis of potassium dynamics in mammalian brain tissue. J Physiol 335:393–426
Heinzle J, Knig P, Salazar RF (2007) Modulation of synchrony without changes in firing rates. Cogn Neurodyn 1:225–235
Hodgkin AL, Katz B (1949) The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol 108:37–77
Jirsa VK (2008) Dispersion and time delay effects in synchronized spike-burst networks. Cogn Neurodyn 2:29–38
Kofuji P, Biedermann B, Siddharthan V, Raap M, Iandiev I, Milenkovic I, Thomzig A, Veh RW, Bringmann A, Reichenbach A (2002) Kir potassium channel subunit expression in retinal glial cells: implications for spatial potassium buffering. Glia 39(3):292–303
Kofuji P, Connors NC (2003) Molecular substrates of potassium spatial buffering in glial cells. Mol Neurobiol 28(2):195–208
Kofuji P, Newman E (2009) Regulation of potassium by glial cells in the central nervous system. In: Parpura V, Haydon P (eds) Springer, New York, pp 151–175
Kuchel PW, Ralston GB (1988) Theory and problems of biochemistry. Schaum’s Outline/McGraw-Hill, New York
Kuffler SW (1967) Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci 168(10):1–21
Kuffler SW, Nicholls JG (1966) The physiology of neuroglial cells. Ergeb Physiol 57:1–90
Kwok HF, Jurica P, Raffone A, van Leeuwen C (2007) Robust emergence of small-world structure in networks of spiking neurons. Cogn Neurodyn 1:39–51
Liu Y, Wang R, Zhang Z, Jiao X (2010) Analysis of stability of neural network with inhibitory neurons. Cogn Neurodyn 4:61–68
Matthews GG (1991) Cellular physiology of nerve and muscle. Blackwell, Boston
Newman EA (1985) Regulation of potassium levels by glial cells in the retina. TINS 8(4):156–159
Newman E, Reichenbach A (1996) The Muller cell: a functional element of the retina. TINS 19(8):307–312
Orkand RK, Nicholls JG, Kuffler SW (1966) Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29(4):788–806
Park EH, Durand DM (2006) Role of potassium lateral diffusion in non-synaptic epilepsy: a computational study. J Theor Biol 238(3):666–682
Purves, D, Augustine, GJ, Fitzpatrick, D, Hall, WC, LaMantia, A-S, McNamara, JO, White, LE (eds) (2008) Neuroscience. 4th edn. Sinauer Associates, Inc, Sunderland Massachusetts, USA
Samson E, Marchand J, Snyder KA (2003) Calculation of ionic diffusion coefficients on the basis of migration test results. Mater Struct 36:156–160
Santhakumar V, Voipio J, Kaila K, Soltesz I (2003) Post-traumatic hyperexcitability is not caused by impaired buffering of extracellular potassium. J Neurosci 23(13):5865–5876
Shi X, Wang Q, Lu Q (2008) Firing synchronization and temporal order in noisy neuronal networks. Cogn Neurodyn 2:195–206
Somjen GG (2002) Ion regulation in the brain: implications for pathophysiology. Neuroscientist 8(3):254–267
Somjen GG, Kager H, Wadman WJ (2008) Computer simulations of neuron-glia interactions mediated by ion flux. J Comput Neurosci 25(2):349–365
Syková E, Chvátal A (1993) Extracellular ionic and volume changes: the role in glia-neuron interaction. J Chem Neuroanat 6:247–260
Syková E (1997) The extracellular space in the CNS: Its regulation, volume and geometry in normal and pathological neuronal function. Neuroscientist 3:28–41
Syková E, Nicholson C (2008) Diffusion in brain extracellular space. Physiol Rev 88(4):1277–1340
Ullah G, Cressman JR Jr, Barreto E, Schiff SJ (2009) The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states. II. Network and glial dynamics. J Comput Neurosci 26(2):171–183
Xu L, Zeng L-H, Wong M (2009) Impaired astrocytic gap junction coupling and potassium buffering in a mouse model of tuberous sclerosis complex. Neurobiol Dis 34:291–299
Zhou W, Jan L (2010) A twist on potassium channel gating. Cell 141:920–922
Acknowledgments
The author is very grateful to Prof. Christine R. Rose (Institute for Neurobiology, Heinrich-Heine-University Duesseldorf) for her inspiring advices on neurobiological aspects of the potassium spatial buffering. The work was supported by the Bundesministerium für Bildung and Forschung (NGFNPlus; FKZ: 01GS08152).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noori, H.R. The impact of the glial spatial buffering on the K+ Nernst potential. Cogn Neurodyn 5, 285–291 (2011). https://doi.org/10.1007/s11571-011-9165-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-011-9165-x