Abstract
Purpose
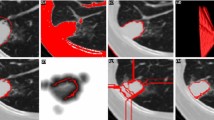
Radiological follow-up of oncology patients requires the quantitative analysis of lesion changes in longitudinal imaging studies, which is time-consuming, requires expertise, and is subject to variability. This paper presents a comprehensive graph-based method for the automatic detection and classification of lesion changes in current and prior CT scans.
Methods
The inputs are the current and prior CT scans and their organ and lesion segmentations. Classification of lesion changes is formalized as bipartite graph matching where lesion pairings are computed by adaptive overlap-based lesion matching. Six types of lesion changes are computed by connected components analysis. The method was evaluated on 208 pairs of lung and liver CT scans from 57 patients with 4600 lesions, 1713 lesion matchings and 2887 lesion changes. Ground-truth lesion segmentations, lesion matchings and lesion changes were created by an expert radiologist.
Results
Our method yields a lesion matching rate accuracy of 99.7% (394/395) and 95.0% (1252/1318) for the lung and liver datasets. Precision and recall are > 0.99 and 0.94 and 0.95 (respectively) for the detection of lesion changes. The analysis of lesion changes helped the radiologist detect 48 missed lesions and 8 spurious lesions in the input ground-truth lesion datasets.
Conclusion
The classification of lesion classification provides the clinician with a readily accessible and intuitive identification and classification of the lesion changes and their patterns in support of clinical decision making. Comprehensive automatic computer-aided lesion matching and analysis of lesion changes may improve quantitative follow-up and evaluation of disease status, assessment of treatment efficacy and response to therapy.


Similar content being viewed by others
References
Eisenhauer EA, Therasse P, Bogaerts J (2009) New response evaluation criteria in solid tumors: revised RECIST guideline (Version 1.1). Eur J Cancer 45(2):228–247
Sosna J (2019) Is RECIST Version 1.1 reliable for tumor response assessment in metastatic cancer. Radiology 290(2):357–358
Joskowicz L, Cohen D, Caplan N, Sosna J (2019) Inter-observer variability of manual contour delineation of structures in CT. Eur Radiol 29(3):1391–1399
Szeskin A, Rochman S, Weis S, Lederman R, Sosna J, Joskowicz L (2023) Liver lesion changes analysis in longitudinal CECT scans by simultaneous deep learning voxel classification with SimU-Net. Med Image Anal 83(1):102675
Shafiei A, Bagheri M, Farhadi, F, Apolo AB, Biassou NM, Folio LR, Jones EC, Summers RM (2021). CT evaluation of lymph nodes that merge or split during the course of a clinical trial: limitations of RECIST 1.1. Radiology: Imaging Cancer 3(3).
Bolme DS, Beveridge, JR. Draper, BA, Lui, YM (2010) Visual object tracking using adaptive correlation filters. In: Proc IEEE conference on computer vision and pattern recognition, pp 2544–2550
Li B, Wu W, Wang Q, Zhang F, Xing J, Yan JS (2019) Evolution of Siamese visual tracking with very deep networks. In: Proc IEEE conference on computer vision and pattern recognition pp 16–20
Teed Z, Deng, J. Raft: Recurrent all-pairs field transforms for optical flow (2020). In: Proc European conference on computer vision, pp 402–419
Ko JP, Betke M (2001) Chest CT: automated nodule detection and assessment of change over time-preliminary experience. Radiology 218(1):267–273
Beyer F, Wormanns D, Novak C, Shen H, Odry BL, Kohl G, Heindel W (2004) Clinical evaluation of a software for automated localization of lung nodules at follow-up CT examinations. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 176(6):829–836
Lee KW, Kim M, Gierada DS, Bae KT (2007) Performance of a computer-aided program for automated matching of metastatic pulmonary nodules detected on follow-up chest CT. Am J Roentgenol 189(5):1077–1081
Koo CW, Anand V, Girvin F, Wickstrom ML, Fantauzzi JP, Bogoni L, Babb JS, Ko JP (2012) Improved efficiency of CT interpretation using an automated lung nodule matching program. Am J Roentgenol 199(1):91–95
Tao C, Gierada DS, Zhu F, Pilgram TK, Wang JH, Bae KT (2009) Automated matching of pulmonary nodules: evaluation in serial screening chest CT. Am J Roentgenol 192(3):624–628
Beigelman-Aubry C, Raffy P, Yang W, Castellino RA, Grenier PA (2007) Computer-aided detection of solid lung nodules on follow-up MDCT screening: evaluation of detection, tracking, and reading time. Am J Roentgenol 189(4):948–955
Hering A, Peisen F, Amaral T, Gatidis S, Eigenter T, Othman TM, J, (2021) Whole-body soft-tissue lesion tracking and segmentation in longitudinal CT imaging studies. Proc Mach Learn Res 143:312–326
Santoro-Fernandes V, Huff D, Scarpelli ML, Perk TG, Albertini MR, Perlman S, Yip SSF, Jeraj R (2021) Development and validation of a longitudinal soft-tissue metastatic lesion matching algorithm. Phys Med Biol 66(15):155017
Moltz JH, Schwier M, Peitgen HO (2009) A general framework for automatic detection of matching lesions in follow-up CT. In: Proc IEEE Int. symp on biomedical imaging, pp 843–846
Moltz JH, D’Anastasi M, Kießling A, Pinto dos Santos D, Schülke C, Peitgen HO (2012) Workflow-centered evaluation of an automatic lesion tracking software for chemotherapy monitoring by CT. Eur Radiol 22:2759–2767
Yan K, Wang X, Lu L, Summers RM (2018) DeepLesion: automated mining of large-scale lesion annotations and universal lesion detection with deep learning. J Med Imag 5(3):036501
Rafael-Palou X, Aubanell A, Bonavita I, Ceresa M, Piella G, Ribas V, Ballester MAG (2021) Re-identification and growth detection of pulmonary nodules without image registration using 3D siamese neural networks. Med Image Anal 67:101823
Cai J, Tang Y, Yan K, Harrison AP, Xiao J, Lin G, Lu L (2021). Deep lesion tracker: monitoring lesions in 4D longitudinal imaging studies. In: Proc. IEEE conf. computer vision and pattern recognition pp 15159–15169
Tang W, Kang H, Zhang H, Yu P, Arnold CW, Zhang R (2022). Transformer lesion tracker. arXiv preprint arXiv:2206.06252
Yip S, Jeraj R (2014) Use of articulated registration for response assessment of individual metastatic bone lesions. Phys Med Biol 59:1501–1514
Kuckertz S, Weiler F, Matusche B, Carsten L, Spies L (2021) A system for fully automated monitoring of lesion evolution over time in multiple sclerosis. Proc SPIE Med Imag 11597:553–558
Kuckertz S, Klein J, Engel C, Geislera B, Krasse S, Heldmann S (2022) Fully automated longitudinal tracking and in-depth analysis of the entire tumor burden: unlocking the complexity. Proc SPIE Med Imag 12033:455–459
Padfield D, Rittscher J, Roysam B (2011) Coupled minimum-cost flow cell tracking for high-throughput quantitative analysis. Med Image Anal 15(4):650–668
Besl PJ, McKay ND (1992). Method for registration of 3-D shapes. In: Sensor fusion IV: control paradigms and data structures. In: Proc. SPIE medical imaging. pp. 586–606
Yushkevich PA, Gao Y, Gerig G (2016) ITK-SNAP: an interactive tool for semi-automatic segmentation of multi-modality biomedical images. In: Proc. 38th Int. Conf. IEEE engineering in medicine and biology society, pp. 3342–3345
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not required for the work reported in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rochman, S., Szeskin, A., Lederman, R. et al. Graph-based automatic detection and classification of lesion changes in pairs of CT studies for oncology follow-up. Int J CARS 19, 241–251 (2024). https://doi.org/10.1007/s11548-023-03000-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-023-03000-2