Abstract
Purpose
Ultrasound (US)-guided percutaneous kidney biopsy is a challenge for interventionists as US artefacts prevent accurate viewing of the biopsy needle tip. Automatic needle tracking and trajectory prediction can increase operator confidence in performing biopsies, reduce procedure time, minimize the risk of inadvertent biopsy bleedings, and enable future image-guided robotic procedures.
Methods
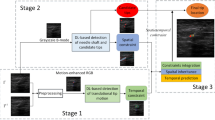
In this paper, we propose a tracking-by-segmentation model with spatial and channel “Squeeze and Excitation” (scSE) for US needle detection and trajectory prediction. We adopt a light deep learning architecture (e.g. LinkNet) as our segmentation baseline network and integrate the scSE module to learn spatial information for better prediction. The proposed model is trained with the US images of anonymized kidney biopsy clips from 8 patients. The contour is obtained using the border-following algorithm and area calculated using Green formula. Trajectory prediction is made by extrapolating from the smallest bounding box that can capture the contour.
Results
We train and test our model on a total of 996 images extracted from 102 short videos at a rate of 3 frames per second from each video. A set of 794 images is used for training and 202 images for testing. Our model has achieved IOU of 41.01%, dice accuracy of 56.65%, F1-score of 36.61%, and root-mean-square angle error of 13.3\(^{\circ }\). We are thus able to predict and extrapolate the trajectory of the biopsy needle with decent accuracy for interventionists to better perform biopsies.
Conclusion
Our novel model combining LinkNet and scSE shows a promising result for kidney biopsy application, which implies potential to other similar ultrasound-guided biopsies that require needle tracking and trajectory prediction.







Similar content being viewed by others
References
Abolhassani N, Patel R, Moallem M (2007) Needle insertion into soft tissue: a survey. Med Eng Phys 29(4):413–431
Apnorton: green’s theorem and area of polygons. https://math.blogoverflow.com/2014/06/04/greens-theorem-and-area-of-polygons
Beigi P, Rohling R, Salcudean SE, Ng GC (2017) Casper: computer-aided segmentation of imperceptible motion—a learning-based tracking of an invisible needle in ultrasound. Int J Comput Assist Radiol Surg 12(11):1857–1866
Board HP (2018) Singapore renal registry annual report 2016
Bradski G (2000) The OpenCV library. Dr. Dobb’s J Softw Tools 25:120–125
Chaurasia A, Culurciello E (2017) Linknet: exploiting encoder representations for efficient semantic segmentation. CoRR http://arxiv.org/abs/1707.03718
Chen LC, Papandreou G, Schroff F, Adam H (2017) Rethinking atrous convolution for semantic image segmentation. arXiv preprint arXiv:1706.05587
Fitzgibbon AW, Fisher RB (1996) A buyer’s guide to conic fitting. DAI Research paper
Foley JD, Van FD, Van Dam A, Feiner SK, Hughes JF, Hughes J, Angel E (1996) Computer graphics: principles and practice, vol 12110. Addison-Wesley Professional, Boston
Hamper UM, Savader BL, Sheth S (1991) Improved needle-tip visualization by color doppler sonography. AJR Am J Roentgenol 156(2):401–402
Hatt CR, Ng G, Parthasarathy V (2015) Enhanced needle localization in ultrasound using beam steering and learning-based segmentation. Comput Med Imaging Graph 41:46–54
He K, Zhang X, Ren S, Sun J (2015) Deep residual learning for image recognition. CoRR http://arxiv.org/abs/1512.03385
Johnson RJ, Feehally J, Floege J (2014) Comprehensive clinical nephrology E-Book. Elsevier Health Sciences, Amsterdam
Kingma DP, Ba J (2014) Adam: a method for stochastic optimization. arXiv preprint arXiv:1412.6980
Mathiassen K, Dall’Alba D, Muradore R, Fiorini P, Elle OJ (2016) Robust real-time needle tracking in 2-D ultrasound images using statistical filtering. IEEE Trans Control Syst Technol 25(3):966–978
Mwikirize C, Nosher JL, Hacihaliloglu I (2018) Convolution neural networks for real-time needle detection and localization in 2D ultrasound. Int J Comput Assist Radiol Surg 13(5):647–657
Neubach Z, Shoham M (2010) Ultrasound-guided robot for flexible needle steering. IEEE Trans Biomed Eng 57(4):799–805
Paszke A, Gross S, Chintala S, Chanan G, Yang E, DeVito Z, Lin Z, Desmaison A, Antiga L, Lerer A (2017) Automatic differentiation in PyTorch. In: NIPS 2017 Autodiff workshop: the future of gradient-based machine learning software and techniques. Long Beach, CA, US
Peng C, Zhang X, Yu G, Luo G, Sun J (2017) Large kernel matters—improve semantic segmentation by global convolutional network. In: Proceedings of the IEEE conference on computer vision and pattern recognition, pp 4353–4361
Roy AG, Navab N, Wachinger C (2018) Concurrent spatial and channel ‘squeeze & excitation’in fully convolutional networks. In: International conference on medical image computing and computer-assisted intervention, pp 421–429. Springer
Suzuki S, Keiichi B (1985) Topological structural analysis of digitized binary images by border following. Comput Vis Graph Image Process 30(1):32–46
Teh CH, Chin RT (1989) On the detection of dominant points on digital curves. IEEE Trans Pattern Anal Mach Intell 11(8):859–872
Tomasi C, Manduchi R (1998) Bilateral filtering for gray and color images. In: Iccv, vol 98, p 2
Vrooijink GJ, Abayazid M, Misra S (2013) Real-time three-dimensional flexible needle tracking using two-dimensional ultrasound. In: 2013 IEEE international conference on robotics and automation (ICRA), pp 1688–1693. IEEE (2013)
Zhao Y, Cachard C, Liebgott H (2013) Automatic needle detection and tracking in 3D ultrasound using an ROI-based RANSAC and Kalman method. Ultrason Imaging 35(4):283–306
Zhao Z, Xu S, Wood B, Tse ZTH (2018) An electromagnetic tracking needle clip: an enabling design for low-cost image-guided therapy. In: 2018 Design of medical devices conference. American Society of Mechanical Engineers, , pp V001T07A010–V001T07A010
Funding
This study was funded by NMRC Bedside & Bench under Grant R-397-000-245-511 awarded to Dr. Hongliang Ren.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. If articles do not contain patient data, the statement should read: This article does not contain patient data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J.Y., Islam, M., Woh, J.R. et al. Ultrasound needle segmentation and trajectory prediction using excitation network. Int J CARS 15, 437–443 (2020). https://doi.org/10.1007/s11548-019-02113-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11548-019-02113-x