Abstract
Purpose
To establish reference ranges for four most commonly used diagnostic measures of craniocervical instability (CCI) in three cervical sagittal positions. This necessitated development of a reliable measurement protocol using upright, dynamic MRI (udMRI), to determine differences in the extent of motion between positions, and whether age and sex correlate with these measures.
Materials and Methods
Deidentified udMRIs of 50 adults, referred for reasons other than CCI, were captured at three positions (maximal flexion, maximal extension and neutral). Images were analyzed, providing measures of basion-axial interval, basion-axial angle, basion-dens interval (BDI) and the Grabb–Oakes line (GOL) for all three positions (12 measures per participant). All measures were independently recorded by a radiologist and neurosurgeon to determine their reliability. Descriptive statistics, correlations, paired and independent t-tests were used. Mean (± 2 SD) identified the reference range for all four measures at each craniocervical position.
Results
The revised measurement protocol produced inter-rater reliability indices of 0.69–0.97 (moderate–excellent). Fifty adults’ (50% male; mean age 41.2 years (± 9.7)) reference ranges for all twelve measures were reported. Except for the BDI and GOL when moving between neutral and full flexion, significant extents of movement were identified between the three craniocervical positions for all four measures (p ≤ 0.005). Only a minor effect of age was found.
Conclusions
This is the first study to provide a rigorous standardized protocol for four diagnostic measures of CCI. Reference ranges are established at mid and ends of sagittal cervical range corresponding to where exacerbations of signs and symptoms are commonly reported.
Similar content being viewed by others
Introduction
Craniocervical stability relies on the integrity of the craniocervical junction which comprises the occiput, atlas and axis that form the occipitoatlantoaxial joint complex [1]. Despite enclosing the brainstem, spinal cord, cranial nerves, C1 and C2 spinal nerve roots and vertebral arteries [1], this junction permits extensive motion particularly in the sagittal and horizontal planes [2]. Its complex kinematics and dynamic stability are conferred by the integrity of specialized ligaments and membranes in addition to joint congruity [3]. Ligamentous laxity and/or bony deficits can result in craniocervical instability (CCI) and allow excessive excursion that jeopardizes the functioning of the central nervous system and cranial nerves [2]. Despite its musculoskeletal origin, CCI can result in debilitating neurological complications.
Consequences of CCI can seriously impact quality of life. Basilar invagination and/or vertebral artery occlusion can result in ventral compression of neural tissues and obstruction of cerebrospinal flow and arterial supply [4, 5]. Reported symptoms although controversial range from headache, vertigo, perceived instability and sensorimotor dysfunction to impaired vision, dyspnea and dysautonomia [5,6,7,8]. However, patients present heterogeneously and may be referred for diagnostic procedures not specific to CCI.
The pathophysiology of CCI is variable. A range of conditions are associated with CCI including, but not limited to, head/neck injury [9], arthritic conditions [10, 11], genetic disorders [12] and heritable disorders of connective tissue [13,14,15]. Despite known associations, the condition-specific prevalence of CCI is inconsistently reported. For example, 10–70% of patients with rheumatoid arthritis are diagnosed with CCI [10, 16], 8–63% of those with Down syndrome [12, 17] and 25–37% with osteogenesis imperfecta [18, 19]. The methodological variability between studies, particularly in the classification of severity and diagnostic criteria of CCI utilized, highlights the difficulties in determining its prevalence and potential impact at both the individual and population levels.
Clinical diagnosis of CCI is confirmed by positive radiological findings. These are well defined for traumatic conditions but less so for nontraumatic etiology. Patients with CCI report symptoms and demonstrate signs of ventral compression during head and neck movements [6, 7, 9, 20,21,22]. Although the common static imaging techniques, such as erect radiography and recumbent MRI, might suffice to detect overt subluxations or neuroanatomical abnormalities [23, 24], signs of mild instability or positional symptoms may be difficult to discern from images taken in a neutral or unloaded head-on-neck position. The presence of provocative positions in CCI, notably flexion, suggests that subluxations and neuroanatomical distortions may be positional and therefore be best diagnosed with functional radiological investigations.
Upright dynamic MRI (udMRI) may substantially enhance diagnosis of CCI [25]. Preliminary evidence suggests that udMRI demonstrates superior diagnostic efficacy compared to static supine or upright imaging. Indeed, plain film and computed tomography imaging provide less accurate and valid measures when assessing the extent of soft tissue pathologies in this area [9, 20, 22]. However, the interpretation of craniocervical measures on udMRI remains unstandardized. Normative ranges in neutral are variously reported [24, 26,27,28], resulting in different cutoff criteria to define the presence of CCI, while normative ranges in maximum flexion and extension are lacking. These gaps render the establishment and validation of the udMRI-specific cutoff values impossible. Reference ranges of CCI measures on udMRI must be determined to enable evidence-based, and objective diagnosis and classification of CCI and to facilitate research addressing management.
The objectives of this exploratory study were fivefold. Using four routine radiological measures, we aimed to determine (i) a reliable measurement protocol to detect the presence of CCI using udMRI, (ii) the reference ranges in three positions (maximal flexion, maximal extension and neutral), (iii) whether differences exist in the extent of motion between neutral to flexion and neutral to extension, (iv) the correlation of age and sex with the measures and v) the proportion of false-positive identifications of CCI.
Methods
Participant recruitment and imaging equipment
Staff of a medical diagnostic imaging center (Western Imaging Group, NSW, Australia) provided deidentified, udMRI images of the craniocervical region of male and female patients 18 years or over. The MRI used in this study was the FONAR UPRIGHT® Multi-Position™ MRI (0.6 Tesla). All were referred for reasons other than CCI. Images of patients who were referred with head or neck trauma, whiplash associated disorder, rheumatological conditions of the craniocervical spine or a hereditary disorder of connective tissue (including but not limited to Ehlers-Danlos Syndrome, Marfan or Loeys-Dietz Syndromes or Osteogenesis Imperfecta) were excluded from the study.
T2 images were captured in the midsagittal plane representing the neutral cervical position (repetition time (TR): 1602 (or 2976 depending on the number of slices used in the original scan) and time to echo (TE): 120), the maximal flexion position (TR: 2082 and TE: 160) and the maximal extension position (TR: 2082 and TE: 160).
Procedure
Three images were extracted for measurement from each patient’s file. These were the midsagittal images captured at maximum cervical flexion, extension and at neutral (mid-way between). The measures chosen to determine craniocervical motion were the (i) basion-axial interval (BAI) also known as the horizontal Harris measurement, (ii) basion-axial angle (BAA) also called the clivo-axial angle, (iii) basion-dens interval (BDI) also known as the vertical Harris measurement and (iv) Grabb–Oakes line (GOL) also known as the Grabb–Mapstone–Oaks measurement. The deidentified scans were reviewed on a medical image viewer (Voyager PACS Intellirad Solutions Pty Ltd, Australia). The program permitted the examiner to mark up the images, automatically calculating the intervals and angles required. The standardized protocol for each measurement including normal reported values is detailed in Figs. 1, 2, 3 and 4.
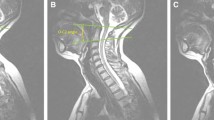
a Schematic representation of the basion-axial interval (BAI), also known as the horizontal Harris measurement. Increase in BAI suggests anterior translation of the cranium relative to the axis [29, 30]. The posterior axial line is drawn through the posteroinferior and the posterior most margins of the vertebral body of C2, regardless of the orientation of the posterosuperior aspect of the odontoid. Reported normal values ≤ 12 mm [29]. b At a neutral cervical spine position, the proposed BAI reference range is 0.5–8.9 mm. c At the maximum cervical flexion position, the proposed reference range is 0.7–10.3 mm. d Maximum cervical extension position, the proposed reference range is − 0.6–7.0 mm. (all images lossy compressed 11%)
a Schematic representation of the basion-axial angle (BAA), also known as the clivo-axial angle (CXA) or clivo-canal angle has suggested normal values > 135° [29]. The Wackenheim line is drawn along the dorsal surface of the lower clivus and extrapolated posteroinferiorly across the superior aspect of the dens tangentially [37]. If the basion curves inferiorly, the line is extended from the middle of the clivus. The posterior axial line is drawn through the posteroinferior and the posterior most margins of the vertebral body of C2, regardless of the odontoid process. The ventral angle (in degrees) of intersection of the two lines is the basion-axial angle. Reduction in BAA suggests increased kyphosis and deformative strain of the brainstem and upper spinal cord [29]. b At a neutral cervical spine position, the proposed BAA reference range is 128–169°. c At the maximum cervical flexion position, the proposed BAA reference range is 126–165°. d Maximum cervical extension position, the proposed BAA reference range is 139–184°. (all images lossy compressed 11%)
a Schematic representation of the basion-dental interval (BDI), which is the minimum distance (in millimeters) from the posteroinferior tip of the basion to the superior aspect of the dens. It has suggested normal values ≤ 12 mm [30]. Increase in BDI or the vertical Harris measurement suggests potential occipito-atlantal instability [26, 38]. b At a neutral cervical spine position, the proposed BDI reference range is 2.0–8.0 mm. c At the maximum cervical flexion position, the proposed BDI reference range is 1.8–8.2 mm. d Maximum cervical extension position, the proposed BDI reference range is 2.4–8.8 mm. (all images lossy compressed 11%)
a Schematic representation of the Grabb–Oakes line (GOL), also known as the Grabb–Mapstone–Oaks measurement is described as a line drawn from the tip of the basion to the infero-posterior aspect of the body of C2. pB-C2 is the perpendicular distance between this line and the ventral cervicomedullary dura [Wholey]. It has suggested normal values of < 9 mm [29]. Increase in length suggests increased risk of ventral compression of the brainstem and upper spinal cord [29, 39]. b At a neutral cervical spine position, the proposed GOL reference range is 4.2–10.2 mm. c At the maximum cervical flexion position, the proposed GOL reference range is 3.8–10.6 mm. d Maximum cervical extension position, the proposed GOL reference range is 2.7–9.1 mm. (all images lossy compressed 11%)
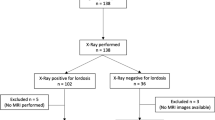
We aimed to utilize 50 participants’ udMRI for this exploratory study, similar sample size to other investigative MRI studies related to craniocervical measures [7, 20]. To determine the inter-tester reliability of the four measures used in the protocol, two of the investigators (senior radiologist ML and senior neurosurgeon PJR) independently measured these on all three images (neutral and maximal flexion and extension). The diagnostic imaging center provided demographic data only to the chief investigator (LLN) to ensure that those performing the measures were blinded to each patient’s age, sex and reason for referral. A priori, we chose to determine the inter-tester reliability (ICCs) of all four measures in each of the three craniocervical positions for the first 20 participants. If any of the twelve ICCs were not acceptable (< 0.7), the assessors would meet to refine the protocol and remeasure the scans and ICCs would be recalculated. If the inter-rater reliability was deemed acceptable, the measures of the radiologist would be used for the remainder of the analysis as this would be consistent with clinical practice.
Visual inspection of the participants’ measures on the BAI, BAA, BDI and GOL in each of the three sagittal cervical positions was undertaken. To determine the proportion of false-positive identification of radiological evidence of CCI in our cohort, traditional cutoff criteria for normality were used based on previous studies [29, 30].
Statistical analysis
All data were analyzed using SPSS (v26 IBM NY USA). Descriptive statistics were used to detail demographic data of age, sex and reason for imaging referral. Intraclass correlation coefficients (ICCs) and their 95% confidence intervals (95% CIs) were used to determine the inter-rater reliability of the four radiological measures in the three cervical spine positions. ICC values of < 0.50 indicate poor reliability, 0.50–0.74 indicate moderate reliability, 0.75–0.90 indicate good reliability, and values greater than 0.90 indicate excellent reliability [31].
Whole cohort means, standard deviations and ranges were determined for each of the radiological measures in all three positions to determine reference values. These cutoff values to differentiate normal from abnormal measures were set at two standard deviations above and below the mean as previously recommended [32, 33]. Paired t-tests and Pearson’s correlation coefficients were used to determine whether relative motion differed between the three craniocervical positions for each of the four radiological measures. That is, was there a significant difference between the extent of motion measured between the neutral and maximal flexion positions, and the neutral and maximal extension positions? We set statistical significance level at p ≤ 0.005 using Bonferroni correction to account for the multiple hypotheses and to minimize type 1 errors. To determine whether age or sex affects these measures, we used independent samples t-tests. Pearson’s correlation (r) was used to determine whether any of the measures were associated with age or sex.
Ethical approval was granted from The University of Sydney Human Research Ethics Committee (Approval #2020/284).
Results
The maximal craniocervical flexion and extension images together with the neutral image in the median sagittal plane of 50 participants were extracted from the series of images captured using udMRI. The mean age (SD, range) of these participants was 41.2 years (± 9.7, 24–69) with no significant age difference between sexes (41.2 years (± 9.3, 24–58) for females; 41.3 years (± 10.3, 27–69) for males (p = 0.98)). Fifty percent of the cohort were male. All data were normally distributed. The reasons for referral for udMRI were as follows: 72% cervical radiculopathy, 24% neck pain and 4% cervical myelopathy.
While the ICCs for the first 20 patients revealed that the BAA and GOL measures demonstrated good to excellent reliability, the BAI in neutral and all BDI measures demonstrated unacceptable reliability (< 0.7). The assessors met to discuss individual differences in interpreting the protocol, resolved these and revised the protocol. All ICCs for the first 20 participants were recalculated. Acceptable inter-tester reliability was now demonstrated for all four outcome measures. When performed on all 50 patients, the ICCs ranged between 0.69 (95%CI 0.45–0.82) and 0.97 (95%CI 0.95–0.98).
The mean, standard deviation, range and the limits of “reference values” for each of the radiological measures in all three craniocervical positions (flexion, extension and neutral) are provided in Table 1. The reference range (also provided in Figs. 1, 2, 3 and 4) is the mean ± 2 standard deviations.
The means of most of the four radiological measures, assessed at neutral and maximal flexion and extension, were significantly different from one another (Table 2). The only measures for which there was no statistical difference (motion between neutral and either flexion or extension) were for the BDI and GOL with negligible mean change when moving from between neutral and flexion.
None of the measures (BAI, BAA, BDI and GOL) were statistically different between male and female participants (all p-values ranged from 0.05 to 0.94) in any of the three cervical positions. Hence, no correlation analyses were performed between the four diagnostic measures and sex. Increasing age was associated with decrease in the extent of some of the measures in flexion and neutral but not in extension. Only the BAI in the neutral position was significantly decreased with age (Table 3).
The number of participants without suspected CCI signs or symptoms (as indicated by the referral) in this study who exhibited results that would be considered diagnostic of this condition as determined by traditional cutoff criteria, that is false positives, are presented in Table 4. Of the 50 participants, two exhibited both abnormal BAA and GOL. These were females, aged 25 and 33 years, and both were referred to imaging for “cervical radiculopathy.”
Discussion
To our knowledge, this study is one of the largest to analyze common measures associated with diagnosis of craniocervical instability (CCI), using upright dynamic MRI. Given that CCI symptoms may only occur or be exacerbated in end-range positions during daily activities [34], it is pertinent to image the craniocervical region in these ranges. We refined and produced a clinically useful protocol for four commonly used measures in mid and end-range flexion and extension, providing reference ranges outside of which abnormalities may be identified.
Prior to collecting the data, we reviewed the literature to find varying descriptions of the measurements. One of the challenges faced by those taking the measures and then by those interpreting them is the variability in protocols used. Much of the unreliability inherent in the measures reported relates to the lack of detail provided by investigators and the anatomical variability of the bony structures. An example of this is the placement of the Wackenheim line. Harris and colleagues report that some measurements use the rostral slope of the posterior clivus [30]. Other investigators interpolate a line that connects the superior and inferior extents of the posterior aspects of the clivus [35]. These versions will generate different BAA measurements (Fig. 5).
This was clearly evidenced by the initial less-than-acceptable inter-rater reliability (ICC < 0.7) initially calculated for two of the four measures. Consultation was undertaken by the two highly experienced examiners to come to a consensus on the protocol for each measure.
The reference ranges as a proxy for “normal” range for the four measures indicate that significant extents of movement occur between the bony landmarks during upper cervical flexion and extension (Table 2). There was a mean 12% increase in the BAI when moving into flexion, a 9% decrease in the BAA moving into extension and a 15% increase in GOL length when moving into extension. These findings further validate the need to use udMRI to fully investigate and understand the mechanics of symptom production in patients with CCI or symptoms associated with end-range craniocervical positioning.
While sex appeared to have no association with relative motion between the skull, atlas and axis, moderate correlations were found between age and motion at the neutral position for BAI, BAA and GOL and in the flexion position for BAI and BAA. BAI and GOL demonstrated that as people age, the intervals decrease, whereas the BAA increased with age in both the flexion and neutral positions.
Our established reference ranges align with the previously recommended cutoff values for the basion-axial interval [29, 30] (horizontal Harris measurement) and the basion-dental interval [30] (vertical Harris measurement). However, our reference ranges for the basion-axial angle [29, 37] (clivo-axial angle) suggest that the > 135° cutoff to indicate normality may result in over-diagnosis of kyphosis resulting in deformation of the brainstem and upper spinal cord. We recorded measures of ≤ 135° in 2–8% (depending on craniocervical position) of our 50 participants who were not referred with symptoms suggestive of CCI. Similarly, our reference ranges indicate that a cutoff value of < 9 mm for the Grabb–Oakes line [29, 39] may overestimate the risk of ventral compression of the brainstem and upper spinal cord. Of our 50 participants, 6–14% recorded measures ≥ 9 mm depending on the cervical position (Table 4).
Strengths of this study include that it is one of the largest studies to investigate reference CCI measures in different positions using udMRI. By including participants across a wide adult age range and of both sexes equally, we were able to identify differences in craniocervical joint mobility that occur naturally, permitting translation of our findings to the general population. Importantly, our study set the statistical significance level at p ≤ 0.005 accounting for family-wise error. Since a potential diagnosis of CCI can be a serious condition with significant financial and psychological ramifications, minimizing false-positive diagnoses was paramount.
Some limitations of this study should be acknowledged. The sample used for this study was retrospectively sourced from an imaging center. The participants were referred for udMRI for reasons other than CCI. While they did not have CCI symptoms, they did have symptoms related to pain or paresthesia for which their craniocervical region was imaged, constituting a possible selection bias. They were not symptom free and may display altered craniocervical kinematics compared to healthy individuals. Despite this, the BAA of 148.6° ± 10.3° in the neutral position agrees with a previous study reporting 148.9° ± 8.4° in a non-symptomatic sample [36]. Future studies should incept a cervical symptom-free cohort from which normative data and consequent cutoff criteria in neutral, and maximal flexion and extension using udMRI can be determined.
Conclusions
This study provides reference values for four commonly used radiological measures used to assist in the diagnosis of craniocervical instability. As it is evident that people can demonstrate false-positive findings on udMRI using more conservative cutoff values, it is imperative to correlate these radiological measures with signs and symptoms of CCI. Special care needs to be taken when interpolating lines on MRI. Using standardized and detailed protocols with high-resolution imaging will further aid this task.
References
Vigo V, Hirpara A, Yassin M, Wang M, Chou D, De Bonis P et al (2020) Immersive surgical anatomy of the craniocervical junction. Cureus 12:e10364. https://doi.org/10.7759/cureus.10364
Lopez AJ, Scheer JK, Leibl KE, Smith ZA, Dlouhy BJ, Dahdaleh NS (2015) Anatomy and biomechanics of the craniovertebral junction. Neurosurg Focus 38:E2. https://doi.org/10.3171/2015.1.FOCUS14807
Tubbs RS, Hallock JD, Radcliff V, Naftel RP, Mortazavi M, Shoja MM et al (2011) Ligaments of the craniocervical junction. J Neurosurg Spine 14:697–709. https://doi.org/10.3171/2011.1.SPINE10612
Smith JS, Shaffrey CI, Abel MF, Menezes AH (2010) Basilar invagination. Neurosurgery 66:39–47
Flanagan MF (2015) The role of the craniocervical junction in craniospinal hydrodynamics and neurodegenerative conditions. Neurol Res Int 2012:794829. https://doi.org/10.1155/2015/794829
Milhorat TH, Bolognese PA, Nishikawa M, McDonnell NB, Francomano CA (2007) Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and chiari malformation type I in patients with hereditary disorders of connective tissue. J Neurosurg Spine 7:601–609. https://doi.org/10.3171/SPI-07/12/601
Henderson FC Sr, Francomano CA, Koby M, Tuchman K, Adcock J, Patel S (2019) Cervical medullary syndrome secondary to craniocervical instability and ventral brainstem compression in hereditary hypermobility connective tissue disorders: 5-year follow-up after craniocervical reduction, fusion, and stabilization. Neurosurg Rev 42:915–936. https://doi.org/10.1007/s10143-018-01070-4
Chu EC, Wong AY, Lee LY (2021) Craniocervical instability associated with rheumatoid arthritis: a case report and brief review. AME Case Rep 5:12. https://doi.org/10.21037/acr-20-131
Johansson BH (2006) Whiplash injuries can be visible by functional magnetic resonance imaging. Pain Res Manag 11:197–199
Carotti M, Salaffi F, Di Carlo M, Sessa F, Giovagnoni A (2019) Magnetic resonance imaging of the craniovertebral junction in early rheumatoid arthritis. Skelet Radiol 48:553–561. https://doi.org/10.1007/s00256-018-3055-9
Seaman SC, Hong S, Dlouhy BJ, Menezes AH (2020) Current management of juvenile idiopathic arthritis affecting the craniovertebral junction. Childs Nerv Syst 36:1529–1538. https://doi.org/10.1007/s00381-019-04469-6
Hankinson TC, Anderson RC (2010) Craniovertebral junction abnormalities in Down syndrome. Neurosurgery 66:32–38
Spiessberger A, Dietz N, Gruter B, Virojanapa J (2020) Ehlers-Danlos syndrome-associated craniocervical instability with cervicomedullary syndrome: comparing outcome of craniocervical fusion with occipital bone versus occipital condyle fixation. J Craniovertebr Junction Spine 11:287–292. https://doi.org/10.4103/jcvjs.JCVJS_166_20
Khandanpour N, Connolly DJA, Raghavan A, Griffiths PD, Hoggard N (2012) Craniospinal abnormalities and neurologic complications of osteogenesis imperfecta: imaging overview. Radiographics 32:2101–2112. https://doi.org/10.1148/rg.327125716
Fuhrhop SK, McElroy MJ, Dietz HC 3rd, MacCarrick GL, Sponseller PD (2015) High prevalence of cervical deformity and instability requires surveillance in Loeys-Dietz syndrome. J Bone Joint Surg Am 97:411–419. https://doi.org/10.2106/JBJS.N.00680
Halla JT, Hardin JG, Vitek J, Alarcón GS (1989) Involvement of the cervical spine in rheumatoid arthritis. Arthritis Rheumatol 32:652–659. https://doi.org/10.1002/anr.1780320522
Taggard DA, Menezes AH, Ryken TC (2000) Treatment of Down syndrome-associated craniovertebral junction abnormalities. J Neurosurg 93:205–213. https://doi.org/10.3171/spi.2000.93.2.0205
Arponen H, Mäkitie O, Haukka J, Ranta H, Ekholm M, Mäyränpää MK et al (2012) Prevalence and natural course of craniocervical junction anomalies during growth in patients with osteogenesis imperfecta. J Bone Miner Res 27:1142–1149. https://doi.org/10.1002/jbmr.1555
Sillence DO (1994) Craniocervical abnormalities in osteogenesis imperfecta: genetic and molecular correlation. Pediatr Radiol 24:427–430. https://doi.org/10.1007/BF02011910
Gupta V, Khandelwal N, Mathuria SN, Singh P, Pathak A, Suri S (2007) Dynamic magnetic resonance imaging evaluation of craniovertebral junction abnormalities. J Comput Assist Tomogr 31:354–359. https://doi.org/10.1097/01.rct.0000238009.57307.26
Vitaz TW, Shields CB, Raque GH, Hushek SG, Moser R, Hoerter N et al (2004) Dynamic weight-bearing cervical magnetic resonance imaging: technical review and preliminary results. South Med J 97:456–461
Suzuki F, Fukami T, Tsuji A, Takagi K, Matsuda M (2008) Discrepancies of MRI findings between recumbent and upright positions in atlantoaxial lesion. Report of two cases. Eur Spine J 17:S304–S307. https://doi.org/10.1007/s00586-008-0595-z
Riascos R, Bonfante E, Cotes C, Guirguis M, Hakimelahi R, West C (2015) Imaging of atlanto-occipital and atlantoaxial traumatic injuries: what the radiologist needs to know. Radiographics 35:2121–2134. https://doi.org/10.1148/rg.2015150035
Joaquim AF, Ghizoni E, Tedeschi H, Appenzeller S, Riew KD (2015) Radiological evaluation of cervical spine involvement in rheumatoid arthritis. Neurosurg Focus 38:E4. https://doi.org/10.3171/2015.1.FOCUS14664
Nouh MR (2019) Imaging of the spine: where do we stand? World J Radiol 11:55–61. https://doi.org/10.4329/wjr.v11.i4.55
Rojas CA, Bertozzi JC, Martinez CR, Whitlow J (2007) Reassessment of the craniocervical junction: normal values on CT. Am J Neuroradiol 28:1819–1823. https://doi.org/10.3174/ajnr.A0660
Chilvers G, Janjua U, Choudhary S (2017) Blunt cervical spine injury in adult polytrauma: incidence, injury patterns and predictors of significant ligament injury on CT. Clin Radiol 72:907–914. https://doi.org/10.1016/j.crad.2017.06.122
Bouchard M, Bauer JM, Bompadre V, Krengel WF (2019) An updated algorithm for radiographic screening of upper cervical instability in patients with Down syndrome. Spine Deform 7:950–956. https://doi.org/10.1016/j.jspd.2019.01.012
Henderson F Sr, Rosenbaum R, Narayanan M, Mackall J, Koby M (2020) Optimizing alignment parameters during craniocervical stabilization and fusion: a technical note. Cureus 12:e7160. https://doi.org/10.7759/cureus.7160
Harris J Jr, Carson G, Wagner L (1994) Radiologic diagnosis of traumatic occipitovertebral dissociation: 1. Normal occipitovertebral relationships on lateral radiographs of supine subjects. Am J Roentgenol 162:881–886. https://doi.org/10.2214/ajr.162.4.8141012
Portney LG, Watkins MP (2009) Foundations of clinical research: applications to practice. Pearson/Prentice Hall Upper Saddle River, New Jersey
AAoO Surgeons (1965) Joint motion: method of measuring and recording. Churchill Livingstone, Chicago
Fairbank J, Pynsent P, Phillips H (1984) Quantitative measurements of joint mobility in adolescents. Ann Rheum Dis 43:288–294. https://doi.org/10.1136/ard.43.2.288
Henderson FC, Wilson WA, Mott S, Mark A, Schmidt K, Berry JK et al (2010) Deformative stress associated with an abnormal clivo-axial angle: a finite element analysis. Surg Neurol Int 1:30. https://doi.org/10.4103/2152-7806.66461
Liu Z, Zhao X, Guan J, Duan W, Goel A, Xia Z et al (2020) Quantitative reduction of basilar invagination: correction target of clivo-axial angle. Clin Spine Surg 33:E386–E390. https://doi.org/10.1097/BSD.0000000000000971
Prezerakos G, Khan F, Davagnanam I, Smith F, Casey A (2019) FM1–7 Cranio-cervical instability in ehlers-danlos syndrome employing upright, dynamic MR imaging; a comparative study. J Neurol Neurosurg Psychiatry 90:e22. https://doi.org/10.1136/jnnp-2019-ABN.69
Smoker WR (1994) Craniovertebral junction: normal anatomy, craniometry, and congenital anomalies. Radiographics 14:255–277. https://doi.org/10.1148/radiographics.14.2.8190952
Wholey MH, Bruwer AJ, Baker HL (1958) The lateral roentgenogram of the neck; with comments on the atlanto-odontoid-basion relationship. Radiology 71:350–356. https://doi.org/10.1148/71.3.350
Grabb PA, Mapstone TB, Oakes WJ (1999) Ventral brain stem compression in pediatric and young adult patients with Chiari I malformations. Neurosurgery 44:520–527. https://doi.org/10.7759/cureus.10364
Acknowledgements
The authors would like to thank the administrative staff of the Western Imaging Group and particularly Senior Radiographer Thanushan Ravinthiran for their assistance in the data acquisition process. Special thanks also to Associate Professor Arnold Wong and The Hong Kong Polytechnic University for their support of the Summer Research Scholars Program allowing their students to participate in this research study.
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
LLN and PJR contributed to conceptualization; LLN, PJR, ML and CC contributed to methodology; PJR and ML contributed to data collection; LLN, TMW, RHYC and CC contributed to data analysis; all authors contributed to writing-original draft preparation and writing-review and editing; LLN and CC supervised the study; and all authors read and approved final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or nonfinancial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Human Research Ethics Committee of the University of Sydney (2020/284).
Consent to participate
Ethical approval was granted as a Waiver of Consent under the National Statement on Ethical Conduct in Human Research (Australia). As such the researchers had no access to identifiable data, and none are included in any part of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nicholson, L.L., Rao, P.J., Lee, M. et al. Reference values of four measures of craniocervical stability using upright dynamic magnetic resonance imaging. Radiol med 128, 330–339 (2023). https://doi.org/10.1007/s11547-023-01588-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-023-01588-8