Abstract
Purpose
Aim of the study was to perform CT texture analysis in patients with gastric cancer (GC) to investigate potential role of radiomics for predicting the occurrence of peritoneal metastases (PM).
Materials and methods
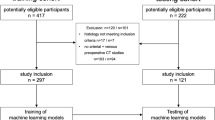
In this single-centre retrospective study, patients with gastric adenocarcinoma and surgically confirmed presence or absence of PM were, respectively, enrolled in group PM and group non-PM. Patients with T1-staging, previous treatment or presence of imaging artifacts were excluded from the study. Pre-operative CT examinations were evaluated. Acquisition protocol consisted of gastric distension with water, pre-contrast and arterial phases on upper abdomen and portal phase on thorax and whole abdomen. Texture analysis was performed on portal phase images: the region of interest was manually drawn along the margins of the primitive lesion on each slice and the volume of interest of the whole tumour was obtained. A total of 38 texture parameters were extracted and analysed. ROC curves were performed on significant texture features (p < 0.05). Multiple logistic regression was conducted on features with the best AUC to identify differentiating variables for both groups.
Results
A total of 90 patients were evaluated (group PM, n = 45; group non-PM, n = 45). T2/T3 tumours were prevalent in group non-PM, T4 was significantly associated with group PM. Significant differences between the two groups were observed for 22/38 texture parameters. Volume and GLRLM_LRHGE showed the greatest AUC in ROC curve analysis (0.737 and 0.734, respectively) and were found to be independent differentiating variables of group PM in the multiple regression analysis (OR 8.44, [95% CI, 1.52–46.8] and OR 18.99 [95% CI, 84–195.31], respectively).
Conclusions
Our preliminary results suggest the potential value of CT texture analysis for predicting the risk of PM from GC, which may be helpful to stratify patients and address them to the most appropriate treatment.



Similar content being viewed by others
Abbreviations
- GC:
-
Gastric cancer
- PM:
-
Peritoneal metastases
- HIPEC:
-
Heated intraperitoneal chemotherapy
- CT:
-
Computed tomography
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under curve
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Stomach Cancer Survival Rates | Gastric Cancer Survival Rates. https://www.cancer.org/cancer/stomach-cancer/detection-diagnosis-staging/survival-rates.html. Accessed 18 May 2020
Hu B, El Hajj N, Sittler S et al (2012) Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol 3:251–261. https://doi.org/10.3978/j.issn.2078-6891.2012.021
Bernards N, Creemers GJ, Nieuwenhuijzen GAP et al (2013) No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Ann Oncol 24:3056–3060. https://doi.org/10.1093/annonc/mdt401
Kodera Y (2018) Surgery with curative intent for stage IV gastric cancer: Is it a reality of illusion? Ann Gastroenterol Surg 2:339–347. https://doi.org/10.1002/ags3.12191
Koemans WJ, Lurvink RJ, Grootscholten C et al (2021) Synchronous peritoneal metastases of gastric cancer origin: incidence, treatment and survival of a nationwide Dutch cohort. Gastric Cancer. https://doi.org/10.1007/s10120-021-01160-1
Thomassen I, van Gestel YR, van Ramshorst B et al (2014) Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 134:622–628. https://doi.org/10.1002/ijc.28373
Bonnot P-E, Piessen G, Kepenekian V et al (2019) Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J Clin Oncol 37:2028–2040. https://doi.org/10.1200/JCO.18.01688
Glehen O, Gilly FN, Arvieux C et al (2010) Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 17:2370–2377. https://doi.org/10.1245/s10434-010-1039-7
Laghi A, Bellini D, Rengo M et al (2017) Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: systematic review and meta-analysis. Radiol med 122:1–15. https://doi.org/10.1007/s11547-016-0682-x
Burbidge S, Mahady K, Naik K (2013) The role of CT and staging laparoscopy in the staging of gastric cancer. Clin Radiol 68:251–255. https://doi.org/10.1016/j.crad.2012.07.015
Iafrate F, Ciolina M, Sammartino P et al (2012) Peritoneal carcinomatosis: imaging with 64-MDCT and 3T MRI with diffusion-weighted imaging. Abdom Imaging 37:616–627. https://doi.org/10.1007/s00261-011-9804-z
Soussan M, Des Guetz G, Barrau V et al (2012) Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy. Eur Radiol 22:1479–1487. https://doi.org/10.1007/s00330-012-2397-2
Bashir U, Siddique MM, Mclean E et al (2016) Imaging heterogeneity in lung cancer: techniques, applications, and challenges. Am J Roentgenol 207:534–543. https://doi.org/10.2214/AJR.15.15864
Lubner MG, Smith AD, Sandrasegaran K et al (2017) CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics 37:1483–1503. https://doi.org/10.1148/rg.2017170056
Rizzo S, Botta F, Raimondi S et al (2018) Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp 2:36. https://doi.org/10.1186/s41747-018-0068-z
Thawani R, McLane M, Beig N et al (2018) Radiomics and radiogenomics in lung cancer: a review for the clinician. Lung Cancer 115:34–41. https://doi.org/10.1016/j.lungcan.2017.10.015
Yip SS, Aerts HJWL (2016) Applications and limitations of radiomics. Phys Med Biol 61:R150–R166. https://doi.org/10.1088/0031-9155/61/13/R150
Rizzo S, Manganaro L, Dolciami M et al (2021) Computed tomography based radiomics as a predictor of survival in ovarian cancer patients: a systematic review. Cancers (Basel) 13:573. https://doi.org/10.3390/cancers13030573
Chetan MR, Gleeson FV (2021) Radiomics in predicting treatment response in non-small-cell lung cancer: current status, challenges and future perspectives. Eur Radiol 31:1049–1058. https://doi.org/10.1007/s00330-020-07141-9
Liu S, He J, Liu S et al (2020) Radiomics analysis using contrast-enhanced CT for preoperative prediction of occult peritoneal metastasis in advanced gastric cancer. Eur Radiol 30:239–246. https://doi.org/10.1007/s00330-019-06368-5
Giganti F, Antunes S, Salerno A et al (2017) Gastric cancer: texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur Radiol 27:1831–1839. https://doi.org/10.1007/s00330-016-4540-y
Huang W, Zhou K, Jiang Y et al (2020) Radiomics nomogram for prediction of peritoneal metastasis in patients with gastric cancer. Front Oncol 10:1416. https://doi.org/10.3389/fonc.2020.01416
Amin MB, Edge S, Greene F et al (2017) AJCC cancer staging manual, 8th edn. Springer
Nioche C, Orlhac F, Boughdad S et al (2018) LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 78:4786–4789. https://doi.org/10.1158/0008-5472.CAN-18-0125
Li Y, Zhang Y, Fang Q et al (2021) Radiomics analysis of [18F]FDG PET/CT for microvascular invasion and prognosis prediction in very-early- and early-stage hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 48:2599–2614. https://doi.org/10.1007/s00259-020-05119-9
Masci GM, Iafrate F, Ciccarelli F et al (2021) Tocilizumab effects in COVID-19 pneumonia: role of CT texture analysis in quantitative assessment of response to therapy. Radiol med. https://doi.org/10.1007/s11547-021-01371-7
Gruzdev IS, Zamyatina KA, Tikhonova VS et al (2020) Reproducibility of CT texture features of pancreatic neuroendocrine neoplasms. Eur J Radiol 133:109371. https://doi.org/10.1016/j.ejrad.2020.109371
Nardone V, Reginelli A, Scala F et al (2019) Magnetic-resonance-imaging texture analysis predicts early progression in rectal cancer patients undergoing neoadjuvant chemoradiation. Gastroenterol Res Pract 2019:8505798. https://doi.org/10.1155/2019/8505798
Jin X, Ai Y, Zhang J et al (2020) Noninvasive prediction of lymph node status for patients with early-stage cervical cancer based on radiomics features from ultrasound images. Eur Radiol 30:4117–4124. https://doi.org/10.1007/s00330-020-06692-1
Wang Z, Chen J-Q (2011) Imaging in assessing hepatic and peritoneal metastases of gastric cancer: a systematic review. BMC Gastroenterol 11:19. https://doi.org/10.1186/1471-230X-11-19
Leiting JL, Grotz TE (2018) Optimizing outcomes for patients with gastric cancer peritoneal carcinomatosis. World J Gastrointest Oncol 10:282–289. https://doi.org/10.4251/wjgo.v10.i10.282
Pasqual EM, Bertozzi S, Londero AP et al (2018) Microscopic peritoneal carcinomatosis in gastric cancer: prevalence, prognosis and predictive factors. Oncol Lett 15:710–716. https://doi.org/10.3892/ol.2017.7442
Kitayama J, Ishigami H, Yamaguchi H et al (2018) Treatment of patients with peritoneal metastases from gastric cancer. Ann Gastroenterol Surg 2:116–123. https://doi.org/10.1002/ags3.12060
Chia DKA, So JBY (2020) Recent advances in intra-peritoneal chemotherapy for gastric cancer. J Gastric Cancer 20:115–126. https://doi.org/10.5230/jgc.2020.20.e15
Desiderio J, Chao J, Melstrom L et al (2017) The thirty-year experience - a meta-analysis of randomized and high quality non-randomized studies of hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of gastric cancer. Eur J Cancer 79:1–14. https://doi.org/10.1016/j.ejca.2017.03.030
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethics approval
This study was approved by the local ethic committee and written informed consent was waived due to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Masci, G.M., Ciccarelli, F., Mattei, F.I. et al. Role of CT texture analysis for predicting peritoneal metastases in patients with gastric cancer. Radiol med 127, 251–258 (2022). https://doi.org/10.1007/s11547-021-01443-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-021-01443-8