Abstract
Purpose
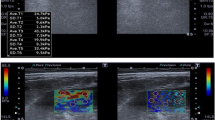
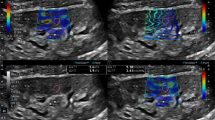
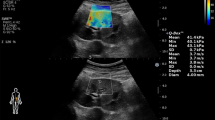
To evaluate the relationship between renal elasticity which was determined with shear wave elastography (SWE) and hemorrhage in patients who undergone percutaneous renal parenchyma biopsy (PRB).
Materials and methods
In total, 60 patients who were performed ultrasound-guided PRB after the B-mode ultrasonography and SWE assessment were recruited in this study. All patients’ serum creatinine, blood urea nitrogen and coagulation tests before PRB were obtained from medical records. The patients were divided into two groups who did and did not develop hemorrhage after PRB. We investigated whether there was any statistically significant difference between the two groups in terms of laboratory findings, B-mode ultrasonographic measurements and SWE measurements.
Results
Of the 60 patients, 23 (38.3%) had post-procedure hemorrhage and 37 (61.7%) had not. Mean hemorrhage size was 17.04 mm (7–50 mm). The mean value of renal cortical shear wave velocity of all patients was 1.91 m/s (0.96–3.57 m/sn). Patients with post-procedure hemorrhage had significantly lower mean shear wave velocity compared with patients with no hemorrhage (p < 0.05). ROC curve analysis suggested that the optimum SWV cutoff point for hemorrhage presence was 1.21 m/sn, with 39.1% sensitivity and 97.3% specificity. There was no other statistically significant demographic, ultrasonographic or laboratory value differences between two groups.
Conclusion
Although shear wave velocities have low sensitivity for hemorrhage after renal biopsy, high specificity and statistically significant difference in hemorrhage and non-hemorrhage group suggest that patients who have lower renal cortical shear wave velocity have a tendency to hemorrhage after PRB.


Similar content being viewed by others
References
Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J et al (2015) US renal data system 2014 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 66:1–305
Waldo B, Korbet SM, Freimanis MG, Lewis EJ (2009) The value of post-biopsy ultrasound in predicting complications after percutaneous renal biopsy of native kidneys. Nephrol Dial Transplant 24:2433–2439
Fuiano G, Mazza G, Comi N, Caglioti A, De Nicola L, Iodice C et al (2000) Current indications for renal biopsy: a questionnaire-based survey. Am J Kidney Dis 35:448–457
Manno C, Strippoli GF, Arnesano L, Bonifati C, Campobasso N, Gesualdo L, Schena FP (2004) Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int 66:1570–1577
Smith EH (1991) Complications of percutaneous abdominal fin-needle biopsy. Radiology 178:253–258
Atwell TD, Spanbauer JC, McMenomy BP, Stockland AH, Hesley GK, Schleck CD et al (2015) The timing and presentation of major hemorrhage after 18,947 image-guided percutaneous biopsies. AJR Am J Roentgenol 205:190–195
Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, Walker TG et al (2012) Standards of practice committee, with cardiovascular and interventional radiological society of Europe (CIRSE) endorsement. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol 23:727–36
Whittier WL, Korbet SM (2004) Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol 15:142–147
Shidham GB, Siddiqi N, Beres JA, Logan B, Nagaraja HN, Shidham SG et al (2005) Clinical risk factors associated with bleeding after native kidney biopsy. Nephrology 10:305–310
Corapi KM, Chen JL, Balk EM, Gordon CE (2012) Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis 60:62–73
Eiro M, Katoh T, Watanabe T (2005) Risk factors for bleeding complications in percutaneous renal biopsy. Clin Exp Nephrol 9:40–45
Torres Muñoz A, Valdez-Ortiz R, González-Parra C, Espinoza-Dávila E, Morales-Buenrostro LE, Correa-Rotter R (2011) Percutaneous renal biopsy of native kidneys: efficiency, safety and risk factors associated with major complications. Arch Med Sci 7:823–831
Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK (2017) Ultrasound elastography: review of techniques and clinical applications. Theranostics 7:1303–1329
Sasaki Y, Hirooka Y, Kawashima H, Ishikawa T, Takeshita K, Goto H (2018) Measurements of renal shear wave velocities in chronic kidney disease patients. Acta Radiol 59:884–890
Hu Q, Wang XY, He HG, Wei HM, Kang LK, Qin GC (2014) Acoustic radiation force impulse imaging for non-invasive assessment of renal histopathology in chronic kidney disease. PLoS ONE 9:e115051
Bob F, Bota S, Sporea I, Sirli R, Popescu A, Schiller A (2015) Relationship between the estimated glomerular filtration rate and kidney shear wave speed values assessed by acoustic radiation force impulse elastography: a pilot study. J Ultrasound Med 34:649–654
Lopez-Novoa JM, Rodriguez-Pena AB, Ortiz A, Martinez-Salgado C, Lopez Hernandez FJ (2011) Etiopathology of chronic tubular, glomerular and renovascular nephropathies: clinical implications. J Transl Med 9:13
Cui G, Yang Z, Zang W, Li B, Sun F, Xu C et al (2014) Evaluation of acoustic radiation force impulse imaging for the clinicopathological typing of renal fibrosis. Exp Ther Med 7:233–235
Wang L, Xia P, Lv K, Han J, Dai Q, Li XM et al (2014) Assessment of renal tissue elasticity by acoustic radiation force impulse quantification with histopathological correlation: preliminary experience in chronic kidney disease. Eur Radiol 24:1694–1699
Goya C, Kilinc F, Hamidi C, Yavuz A, Yildirim Y, Cetincakmak MG et al (2015) Acoustic radiation force impulse imaging for evaluation of renal parenchyma elasticity in diabetic nephropathy. AJR Am J Roentgenol 204:324–329
Kriegshauser JS, Patel MD, Young SW, Chen F, Eversman WG, Chang YH (2015) Risk of bleeding after native renal biopsy as a function of preprocedural systolic and diastolic blood pressure. J Vasc Interv Radiol 26:206–212
Munger KA, Kost CK Jr, Brenner BM, Maddox DA (2012) The renal circulations and glomerular ultrafiltration. In: Taal MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM (eds) Brenner and Rector’s the kidney, 9th edn. Elsevier, Philadelphia, pp 94–137
El Nahas M (2005) The global challenge of chronic kidney disease. Kidney Int 68:2918–2929
Guo LH, Xu HX, Fu HJ, Peng A, Zhang YF, Liu LN (2013) Acoustic radiation force impulse imaging for noninvasive evaluation of renal parenchyma elasticity: preliminary findings. PLoS ONE 8:e68925
Asano K, Ogata A, Tanaka K, Ide Y, Sankoda A, Kawakita C et al (2014) Acoustic radiation force impulse elastography of the kidneys Is shear wave velocity affected by tissue fibrosis or renal blood flow? J Ultrasound Med 33:793–801
Liu X, Li N, Xu T, Sun F, Li R, Gao Q et al (2017) Effect of renal perfusion and structural heterogeneity on shear wave elastography of the kidney: an in vivo and ex vivo study. BMC Nephrol 18:265
Gennisson JL, Grenier N, Combe C, Tanter M et al (2012) Supersonic shear wave elastography of in vivo pig kidney: influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med Biol 38:1559–1567
Amador C, Urban M, Kinnick R, Chen S, Greenleaf JF (2013) In vivo swine kidney viscoelasticity during acute gradual decrease in renal blood flow: pilot study. Rev Ing Biomed 7:68–78
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Çildağ, M.B., Gök, M. & Abdullayev, O. Pre-procedural shear wave elastography on prediction of hemorrhage after percutaneous real-time ultrasound-guided renal biopsy. Radiol med 125, 784–789 (2020). https://doi.org/10.1007/s11547-020-01176-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-020-01176-0