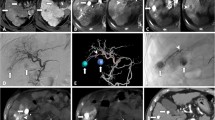
Abstract
Objective
To evaluate the prognostic value of sequential dual-phase CBCT (DP-CBCT) imaging performed during degradable starch microsphere TACE (DSM-TACE) session in predicting the HCC’s response to treatment, evaluate with modify response evaluation criteria in solid tumours (mRECIST) at 1-month multi-detector CT (MDCT) follow-up.
Materials and methods
Between January and May 2018, 24 patients (68.5 ± 8.5 year [45–85]) with HCC lesions (n = 96 [average 4/patient]) were prospectively enrolled. Imaging assessment included: pre-procedural MDCT, intra-procedural DP-CBCT performed before first and second DSM-TACEs and 1-month follow-up MDCT. Lesions’ attenuation/pseudo-attenuation was defined as average value measured on ROIs (HU for MDCT; arbitrary unit called HU* for CBCT). Lesions’ attenuation modification was correlated with the post-procedural mRECIST criteria at 1-month MDCT.
Results
Eighty-two DSM-TACEs were performed. Lesion’s attenuation values were: pre-procedural MDCT arterial phase (AP) 107.00 HU (CI 95% 100.00–115.49), venous phase (VP) 85.00 HU (CI 95% 81.13–91.74); and lesion’s pseudo-attenuation were: first CBCT-AP 305.00 HU* (CI 95% 259.77–354.04), CBCT-VP 155.00 HU* (CI 95% 135.00–163.34). For second CBCT were: -AP 210.00 HU* (CI 95% 179.47–228.58), -VP 141.00 HU* (CI 95% 125.47–158.11); and for post-procedural MDCT were: -AP 95.00 HU (CI 95% 81.35–102.00), -VP 83.00 HU (CI 95% 78.00–88.00). ROC curve analysis showed that a higher difference pseudo-attenuation between first and second DP-CBCTs is related to treatment response. The optimal cut-off value of the difference between first and second CBCT-APs to predict complete response, objective response (complete + partial response) and overall disease control (objective response + stable disease) were > 206 HU* (sensitivity 80.0%, specificity 81.7%), > 72 HU* (sensitivity 79.5%, specificity 83.0%) and > − 7 HU* (sensitivity 91.6%, specificity 65.4%), respectively.
Conclusions
DP-CBCT can predict intra-procedurally, by assessing lesion pseudo-attenuation modification, the DSM-TACE 1-month treatment outcome.




Similar content being viewed by others
Abbreviations
- HCC:
-
Hepato-cellular carcinoma
- DSM-TACE:
-
Degradable starch microsphere trans-arterial chemo-embolization
- MDCT:
-
Multi-detector computed tomography
- CBCT:
-
Cone-beam computer tomography
- DSA:
-
Digital subtraction angiography
- HU:
-
Hounsfield units
- ROIs:
-
Region of interest
- mRECIST:
-
Modify response evaluation criteria in solid tumours
- ROC:
-
Receiver operating characteristics
- AUC:
-
Area under the curve
- CR:
-
Complete response
- PR:
-
Partial response
- OR:
-
Objective response
- SD:
-
Stable disease
- DC:
-
Disease control
- PD:
-
Progressive disease
References
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56(4):908–943
Orlacchio A et al (2015) Downstaging disease in patients with hepatocellular carcinoma outside up-to-seven criteria: strategies using degradable starch microspheres transcatheter arterial chemo-embolization. World J Hepatol 7(12):1694–1700
Lucatelli P et al (2017) Single injection dual phase CBCT technique ameliorates results of trans-arterial chemoembolization for hepatocellular cancer. Transl Gastroenterol Hepatol 2:83
Lucatelli P et al (2017) Comparison of image quality and diagnostic performance of cone-beam CT during drug-eluting embolic transarterial chemoembolization and multidetector CT in the detection of hepatocellular carcinoma. J Vasc Interv Radiol 28(7):978–986
Lucatelli P et al (2015) Impact of 3D rotational angiography on liver embolization procedures: review of technique and applications. Cardiovasc Interv Radiol 38(3):523–535
Miyayama S et al (2016) Efficacy of automated tumor-feeder detection software using cone-beam computed tomography technology in transarterial embolization through extrahepatic collateral vessels for malignant hepatic tumors. Hepatol Res 46(2):166–173
Minami Y et al (2014) Tracking navigation imaging of transcatheter arterial chemoembolization for hepatocellular carcinoma using three-dimensional cone-beam CT angiography. Liver Cancer 3(1):53–61
Lee IJ et al (2015) Cone-beam computed tomography (CBCT) hepatic arteriography in chemoembolization for hepatocellular carcinoma: performance depicting tumors and tumor feeders. Cardiovasc Interv Radiol 38(5):1218–1230
Deschamps F et al (2010) Computed analysis of three-dimensional cone-beam computed tomography angiography for determination of tumor-feeding vessels during chemoembolization of liver tumor: a pilot study. Cardiovasc Interv Radiol 33(6):1235–1242
Schernthaner RE et al (2016) Improved visibility of metastatic disease in the liver during intra-arterial therapy using delayed arterial phase cone-beam CT. Cardiovasc Interv Radiol 39(10):1429–1437
Muller K et al (2017) The role of dual-phase cone-beam CT in predicting short-term response after transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 28(2):238–245
Vogl TJ et al (2016) Intraprocedural blood volume measurement using C-arm CT as a predictor for treatment response of malignant liver tumours undergoing repetitive transarterial chemoembolization (TACE). Eur Radiol 26(3):755–763
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30(1):52–60
Syha R et al (2016) C-arm computed tomography and volume perfusion computed tomography (VPCT)-based assessment of blood volume changes in hepatocellular carcinoma in prediction of midterm tumor response to transarterial chemoembolization: a single center retrospective trial. Cancer Imaging 16(1):30
Syha R et al (2016) Parenchymal blood volume assessed by C-arm-based computed tomography in immediate posttreatment evaluation of drug-eluting bead transarterial chemoembolization in hepatocellular carcinoma. Investig Radiol 51(2):121–126
Loffroy R et al (2013) Intraprocedural C-arm dual-phase cone-beam CT: Can it be used to predict short-term response to TACE with drug-eluting beads in patients with hepatocellular carcinoma? Radiology 266(2):636–648
Lencioni R, Petruzzi P, Crocetti L (2013) Chemoembolization of hepatocellular carcinoma. Semin Interv Radiol 30(1):3–11
Facciorusso A et al (2015) Transarterial chemoembolization: evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol 7(16):2009–2019
Sieghart W, Hucke F, Peck-Radosavljevic M (2015) Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol 62(5):1187–1195
Tavernier J et al (2014) Comparison of two transarterial chemoembolization strategies for hepatocellular carcinoma. Anticancer Res 34(12):7247–7253
Schicho A et al (2017) Transarterial chemoembolization (TACE) with degradable starch microspheres (DSM) in hepatocellular carcinoma (HCC): multi-center results on safety and efficacy. Oncotarget 8(42):72613–72620
Seki A, Hori S (2012) Switching the loaded agent from epirubicin to cisplatin: salvage transcatheter arterial chemoembolization with drug-eluting microspheres for unresectable hepatocellular carcinoma. Cardiovasc Interv Radiol 35(3):555–562
Chung JW (2017) Is scheduled second chemoembolization necessary for early stage hepatocellular carcinoma showing complete response after first chemoembolization? Clin Mol Hepatol 23(1):31–33
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lucatelli, P., De Rubeis, G., Basilico, F. et al. Sequential dual-phase cone-beam CT is able to intra-procedurally predict the one-month treatment outcome of multi-focal HCC, in course of degradable starch microsphere TACE. Radiol med 124, 1212–1219 (2019). https://doi.org/10.1007/s11547-019-01076-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-019-01076-y