Abstract
Purpose
To evaluate whether perfusion and diffusion parameters from staging MR in ovarian cancer (OC) patients may predict the presence of residual tumor at surgery and the progression-free survival (PFS) in 12 months.
Materials and methods
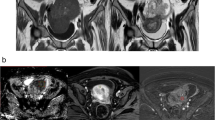
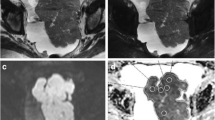
Patients who are from a single institution, candidate for OC to cytoreductive surgery and undergoing MR for staging purposes were included in this study. Inclusion criteria were: preoperative MR including diffusion-weighted imaging (DWI) and perfusion dynamic contrast-enhanced (DCE) sequence; cytoreductive surgery performed within a month from MR; and minimum follow-up of 12 months. Patients’ characteristics including the presence of residual tumor at surgery (R0 or R1) and relapse within 12 months from surgery were recorded. DWI parameters included apparent diffusion coefficient (ADC) of the largest ovarian mass (O-ADC) and normalized ovarian ADC as a ratio between ovarian ADC and muscle ADC (M-ADC). DCE quantitative parameters included were descriptors of tumor vascular properties such as forward and backward transfer constants, plasma volume and volume of extracellular space. Statistical analysis was performed, and p values < 0.05 were considered significant.
Results
Forty-nine patients were included. M-ADC showed a slightly significant association with the presence of residual tumor at surgery. None of the other functional parameters showed either difference between R0 and R1 patients or association with PFS in the first 12 months.
Conclusions
This preliminary study demonstrated a slightly significant association between normalized ovarian ADC and the presence of residual tumor at surgery. The other perfusion and diffusion parameters were not significant for the endpoints of this study.


Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics. CA Cancer J Clin 69(1):7–34
Vergote I, Tropé CG, Amant F et al (2010) Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363(10):943–953
du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J (2009) Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115:1234–1244
Forstner R, Sala E, Kinkel K, Spencer JA (2010) ESUR guidelines: ovarian cancer staging and follow-up. European Society of Urogenital Radiology. Eur Radiol 20(12):2773–2780
Michielsen K, Dresen R, Vanslembrouck R et al (2017) Diagnostic value of whole body diffusion-weighted MRI compared to computed tomography for pre-operative assessment of patients suspected for ovarian cancer. Eur J Cancer 83:88–98
Zahra MA, Tan LT, Priest AN et al (2009) Semiquantitative and quantitative dynamic contrast-enhanced magnetic resonance imaging measurements predict radiation response in cervix cancer. Int J Radiat Oncol Biol Phys 74(3):766–773
Padhani AR, Liu G, Koh DM et al (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Thomassin-Naggara I, Siles P, Balvay D, Cuenod CA, Carette MF, Bazot M (2013) MR perfusion for pelvic female imaging. Diagn Interv Imaging 94(12):1291–1298
Park SB (2016) Functional MR imaging in gynecologic malignancies: current status and future perspectives. Abd Imaging 41:2509–2523
Rizzo S, Buscarino V, Origgi D et al (2016) Evaluation of diffusion-weighted imaging (DWI) and MR spectroscopy (MRS) as early response biomarkers in cervical cancer patients. Radiol Med 121(11):838–846
Levy A, Medjhoul A, Caramella C et al (2011) Interest of diffusion-weighted echo-planar MR imaging and apparent diffusion coefficient mapping in gynecological malignancies: a review. J Magn Reson Imaging JMRI 33(5):1020–1027
Sala E, Rockall A, Rangarajan D, Kubik-Huch RA (2010) The role of dynamic contrast-enhanced and diffusion weighted magnetic resonance imaging in the female pelvis. Eur J Radiol 76(3):367–385
Bellomi M, Rizzo S, Travaini LL et al (2007) Role of multidetector CT and FDG-PET/CT in the diagnosis of local and distant recurrence of resected rectal cancer. Radiol Med 112(5):681–690
Suidan RS, Ramirez PT, Sarasohn DM et al (2014) A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol 134(3):455–461
Suidan RS, Ramirez PT, Sarasohn DM et al (2017) A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol Oncol 145(1):27–31
Rizzo S, Botta F, Raimondi S et al (2018) Radiomics of high-grade serous ovarian cancer: association between quantitative CT features, residual tumour and disease progression within 12 months. Eur Radiol 28(11):4849–4859
Aletti GD, Garbi A, Messori P et al (2017) Multidisciplinary approach in the management of advanced ovarian cancer patients: a personalized approach. Results from a specialized ovarian cancer unit. Gynecol Oncol. 144(3):468–473
Rizzo S, Calareso G, De Maria F et al (2013) Gynecologic tumors: how to communicate imaging results to the surgeon. Cancer Imaging 13(4):611–625
Mohaghegh P, Rockall AG (2012) Imaging strategy for early ovarian cancer: characterization of adnexal masses with conventional and advanced imaging techniques. Radiographics 32:1751–1773
Schmeel FC (2018) Variability in quantitative diffusion-weighted MR imaging (DWI) across different scanners and imaging sites: is there a potential consensus that can help reducing the limits of expected bias? Eur Radiol 29(5):2243–2245. https://doi.org/10.1007/s00330-018-5866-4
Vargas HA, Barrett T, Sala E (2013) MRI of ovarian masses. J Magn Reson Imaging 37:265–281
Lindgren A, Anttila M, Rautiainen S et al (2017) Primary and metastatic ovarian cancer: Characterization by 3.0T diffusion-weighted MRI. Eur Radiol 27(9):4002–4012
Gonzalez Hernando C, Esteban L, Canas T, Van den Brule E, Pastrana M (2010) The role of magnetic resonance imaging in oncology. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex 12(9):606–613
Verma S, Turkbey B, Muradyan N et al (2012) Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol 198(6):1277–1288
Rizzo S, Femia M, Buscarino V et al (2018) Endometrial cancer: an overview of novelties in treatment and related imaging keypoints for local staging. Cancer Imaging 18(1):45
Thomassin-Naggara I, Toussaint I, Perrot N et al (2011) Characterization of complex adnexal masses: value of adding perfusion- and diffusion-weighted MR imaging to conventional MR imaging. Radiology 258(3):793–803
Rizzo S, Kalra MK, Schmidt B et al (2005) CT images of abdomen and pelvis: effect of nonlinear three-dimensional optimized reconstruction algorithm on image quality and lesion characteristics. Radiology 237(1):309–315
Tofts PS, Brix G, Buckley DL et al (1999) Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging JMRI 10(3):223–232
Fusco R, Sansone M, Granata V et al (2018) Diffusion and perfusion MR parameters to assess preoperative short-course radiotherapy response in locally advanced rectal cancer: a comparative explorative study among Standardized Index of Shape by DCE-MRI, intravoxel incoherent motion- and diffusion kurtosis imaging-derived parameters. Abdom Radiol (NY). https://doi.org/10.1007/s00261-018-1801-z
Romero G, Foster BR, Pettersson DR, Fung AW, Guimaraes AR, Coakley FV (2016) Endorectal multiparametric MRI of the prostate: incremental effect of perfusion imaging on biopsy target identification. Clin Imaging 40(3):553–557
Sala E, Kataoka MY, Priest AN et al (2012) Advanced ovarian cancer: multiparametric MR imaging demonstrates response- and metastasis-specific effects. Radiology 263(1):149–159
Acknowledgements
We hereby thank Dr. Antonello Vidiri for sharing his knowledge about the use of the software tool for quantitative DCE analysis. We also thank Lorenzo Viarengo and Carmelo Parisi, GE employees, for their kind assistance during the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee (IEO-CCM Ethical Committee; R 466/16-IEO482) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Piano, F., Buscarino, V., Maresca, D. et al. Do DWI and quantitative DCE perfusion MR have a prognostic value in high-grade serous ovarian cancer?. Radiol med 124, 1315–1323 (2019). https://doi.org/10.1007/s11547-019-01075-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-019-01075-z