Abstract
Purpose
This study was done to assess the effectiveness and advantages of computed tomography (CT) fluoroscopy as a guide for locating and treating lesions that are not amenable to ultrasound (US) guidance, and to evaluate the CT signs of immediate technical success and the short-term results.
Materials and methods
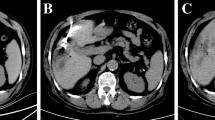
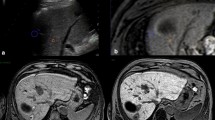
Over the past year, we selected 14 patients (four women and ten men; mean age 73, range 61–83 years) out of 103 candidates for hepatic radiofrequency ablation (RFA). The 14 lesions comprised seven residual tumours after combined embolisation and US-guided RFA of a large hepatocellular carcinoma (HCC), which were indistinguishable from necrosis or surrounding healthy parenchyma; two HCC nodules in locations that were inaccessible by US; five metastases (two from renal carcinoma, two from colorectal adenocarcinoma and one from lung carcinoma), of which one could not be distinguished from the surrounding healthy parenchyma on US and four were inaccessible by US. Lesion diameters were between 1.4 and 3.5 cm. The procedures were performed in the CT room with anaesthesiological assistance using a coaxial LeVeen needle electrode (14 gauge, 2-to 4-cm array diameter). Immediate technical success was evaluated by multidetector CT (MDCT), and follow-up was carried out with MDCT at 3 and 6 months and yearly thereafter.
Results
Immediate technical success was obtained in 13/14 patients; one case required further placement of the electrode due to incomplete ablation of a hypervascular lesion. In 2/3 metastatic lesions with portal vein supply, there were no recurrences at 3 and 6 months; in 1/3, we observed disease progression, with the appearance of additional nodules at 6 months. The two metastases with arterial supply showed no signs of recurrence at 3 months; one case developed a recurrence along the ablation margin, with the appearance of satellite nodules at 6 months. In two HCC nodules, there was immediate technical success and no recurrence at 3 and 6 months. Of the seven residual tumours of HCC, all treated with immediate technical success, we observed disease progression, with the appearance of satellite nodules at 3 months in one case, at 6 months in another and at 12 months in another; 3/7 patients were free of disease at 12-month follow-up; 1/7 died 5 months later due to causes unrelated to the procedure.
Conclusions
CT fluoroscopy is overcoming the limitations of CT in locating and treating lesions with different hepatic vascularisation and those unamenable to US; furthermore, it reduces the length of the procedure, thanks to the faster and more accurate placement of the needle electrode. MDCT proved to be a reliable method in the assessment of immediate and short-term results of RFA.
Riassunto
Obiettivo
Valutare l’efficacia e i vantaggi della guida fluoro-TC nel localizzare e trattare lesioni non raggiungibili o non fattibili con guida ecografica e valutarne i segni TC di successo tecnico immediato e a breve distanza di tempo.
Materiali e metodi
Nell’ultimo anno abbiamo selezionato 14/103 pazienti (4 femmine e 10 maschi) candidati a un trattamento di radiofrequenza (RF) epatica, età media 73 anni (range 61–83) portatori di 14 lesioni epatiche: 7 residui di grosso HCC, esito di terapia combinata mediante embolizzazione e RF eco-guidata non distinguibili dalla necrosi o dal parenchima epatico sano circostante; 2 noduli di HCC non raggiungibili con guida ecografica per sede; 5 metastasi (2 da carcinoma del rene, 2 da adenocarcinoma del colon-retto e 1 da carcinoma del polmone), di cui 1 non distinguibile dal parenchima sano circostante all’ecografia e 4 non raggiungibili per sede. Le lesioni presentavano diametro compreso tra 1,4 e 3,5 cm. Le procedure sono state espletate in sala TC in assistenza anestesiologica con ago elettrodo di LeVeen coassiale (14 gauge, diametro apertura uncini da 2 a 4 cm). Il successo tecnico immediato è stato valutato con TC multistrato (TCMS) post-procedura; il follow-up è stato espletato mediante TCMS a 3, 6 mesi e successivamente annualmente.
Risultati
È stato ottenuto successo tecnico immediato in 13/14 pazienti; in 1 caso e stata necessaria un’infissione ulteriore per parziale ablazione di lesione ipervascolarizzata. Nelle 3 lesioni metastatiche a prevalente vascolarizzazione veno-portale si è documentato successo tecnico immediato e assenza di recidiva a 3 e 6 mesi in 3 casi; un paziente ha sviluppato a 6 mesi una progressione di malattia per comparsa di altre lesioni ripetitive. Le 2 metastasi a prevalente vascolarizzazione arteriosa, non hanno evidenziato a 3 mesi segni TC di recidiva; a 6 mesi in 1 caso si è documentata una recidiva lungo il margine di ablazione e comparsa di microlesioni satelliti. I 2 noduli di HCC trattati hanno ottenuto entrambi successo tecnico immediato e assenza di recidiva a 3 e 6 mesi dal trattamento. Dei 7 residui di HCC, tutti trattati con successo tecnico immediato, 1/7 ha avuto ripresa di malattia per comparsa di noduli satelliti a 3 mesi, 1/7 a 6 mesi e 1/7 a 12 mesi; 3/7 sono liberi da malattia al followup a 12 mesi; 1 è deceduto prima dei 6 mesi per cause non correlate alla procedura.
Conclusioni
La guida fluoro-TC ha attualmente superato i limiti della TC nel localizzare e trattare lesioni a differente vascolarizzazione epatica e quelle non fattibili sotto guida ecografica; inoltre ha ridotto i tempi di procedura per il più veloce ed accurato posizionamento dell’ago-elettrodo. La TCMS si è dimostrata metodica affidabile nella valutazione dei risultati della RF immediati e a breve distanza.
Similar content being viewed by others
References/Bibliografia
Curley SA, Izzo F, Lee ME et al (2000) Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg 232:381–391
De Baere T, Elias D, Dromain C et al (2000) Radiofrequency ablation of 100 hepatic metastases with a mean followup of more than 1 year. AJR Am J Roentgenol 175:1619–1625
Livraghi T, Goldberg SN, Solbiati L et al (2001) Percutaneous radio-frequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology 220:145–149
Sato M, Watanabe Y, Tokui K et al (2000) CT-guided treatment of ultrasonically invisible hepatocellular carcinoma. Am J Gastroenterol 95:2102–2106
Schweiger GD, Brown BP, Pelsang RE et al (2000) CTfluoroscpy: tecnique and utility in guiding biopsies of transiently enhancing hepatic masses. Abdominal Imaging 25:81–85
Oturai AB, Jensen K, Eriksen J, Madsen F (1996) Neurosurgery for trigeminal neuralgia: comparison of alcohol block, neurectomy and radiofrequency coagulation. Clin J Pain 12:311–315
Dupuy DE, Zagoria RJ, Akerley W et al (2000) Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 174:57–59
Rosenthal DI, Hornicek FJ, Wolfe MW et al (1998) Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am 80:815–821
Dupuy DE, Goldberg SN (2001) Image-guided radiofrequency tumor ablation: challenges and opportunities — Part II. J Vasc Interv Radiol 12:1135–1148
Gazelle SG, Goldberg SN, Solbiati L, Livraghi T (2000) Tumor ablation with radiofrequency energy. Radiology 217:633–646
Lagana D, Carrafiello G, Mangini M et al (2006) Radiofrequency ablation of primary and metastatic lung tumors: preliminary experience with a single center device. Surg Endosc 20:1262–1267
Carrafiello G, Lagana D, Mangini M et al (2006) Treatment of secondary hyperparathyroidism with ultrasonographically guided percutaneous radiofrequency thermoablation. Surg Laparosc Endosc Percutan Tech 16:112–116
Goldberg SN, Dupuy DE (2001) Image-guided radiofrequency tumor ablation: challenges and opportunities-part I. J VIR 12:1021–1032
Kay GN, Plumb VJ (1996) The present role of radiofrequency catheter ablation in the management of cardiac arrhythmias. Am J Med 100:344–356
Wagshal AB, Pires LA, Huang SK (1995) Management of cardiac arrhythmias with radiofrequency catheter ablation. Arch Intern Med 155:137–147
Rhim H, Goldberg SN, Dodd GD et al (2001) Essential techniques for successful radiofrequency thermal ablation of malignant hepatic tumors. RadioGraphics 21:17–35
Shankar S, Bhargava P, Habib F et al (2005) Transpulmonary CTguided radiofrequency ablation of liver metastasis. Cardiovasc Intervervent Radiol 28:481–484
Bruix J, Sherman M, Llovet JM et al (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–430
Solbiati L, Ierace T, Tonolini M, Cova L (2004) Guidance and monitoring of radiofrequency liver tumor ablation with contrast-enhanced ultrasound. Eur J Radiol 51:S19–S23
Toyoda M, Kakizaki S, Horiuchi K et al (2006) Computed tomographyguided transpulmonary radiofrequency ablation for hepatocellular carcinoma located in hepatic dome. World J Gastroenterol 12:608–611
Shankar S, Tuncali K, VanSonnenberg E et al (2002) Myoglobinemia after CT-guided radiofrequency ablation of hepatic metastasis. AJR Am J Roentgenol 178:359–361
Antoch G, Kuehl H, Vogt FM et al (2002) Value of CT volume imaging for optimal placement of radiofrequency ablation probes in liver lesions. J Vasc Interv Radiol 13:1155–1161
Seror O, Haddar D, N’Kontchou G et al (2005) Radiofrequency ablation for the treatment of liver tumors in the caudate lobe. J Vasc Interv Radiol 16:981–990
Bissoli E, Bison L, Giuolis C, Fabbris R (2003) Multislice CT-fluoroscopy: technical principles, clinical applications and dosimetry. Radiol Med 106:201–212
Wagner LK (2000) CT-fluoroscopy: another advancement with additional challenges in radiation management. Radiology 216:9–10
Irie T, Kajitani M, Yuji I (2001) CT fluoroscopy-guided intervention: marked reduction of scatter radiation dose to the physician’s hand by use a of lead plate and improved I-I device. SCVIR 12:1417–1421
Silverman SG, Tuncali K, Adams DF et al (1999) CT fluoroscopy-guided abdominal interventions: techniques, results, and radiation exposure. Radiology 212:673–681
Paulson EK, Sheafor DH, Enterline DS et al (2001) CT fluoroscopy-guided interventional procedures: techniques and radiation dose to radiologists. Radiology 220:161–167
Nawfel RD, Judy PF, Silverman SG et al (2000) Patient and personnel exposure during CT fluoroscopy-guided interventional procedures. Radiology 216:180–184
Stoeckelhuber BM, Leibecke T, Schulz E et al (2005) Radiation dose to the radiologist’s during continous CT fluoroscopy-guided interventions. Cardiovasc Intervent Radiol 28:589–594
Dromain C, de Baere T, Elias D et al (2002) Hepatic tumors treated with percutaneous radiofrequency ablation: CT and MR imaging follow-up. Radiology 223:255–262
Merkle EM, Boll DT, Boaz T (1999) MRI-guided radiofrequency thermal ablation of inplanted VX2 liver tumors in a rabbit model: demonstration of feasibility at 0.2 T. Magn Reson Med 42:141–149
McLoughlin RF, Saliken JF, McKinnon G et al (1995) CT of the liver after cryotherapy of hepatic metastases: imaging findings. AJR Am J Roentgenol 165:329–332
Cha CH, Lee FT Jr, Gurney JM et al (2000) CT versus sonography for monitoring radiofrequency ablation in a porcine liver. AJR Am J Roentgenol 175:705–711
Crowley JD, Shelton J, Iverson AJ et al (2001) Laparoscopic and computed tomography-guided percutaneous radiofrequency ablation of renal tissue: acute and chronic effects in an animal model. Urology 57:976–980
Raman SS, Lu DS, Vodopich DJ et al (2000) Creation of radiofrequency lesions in a porcine model: correlation with sonography, CT, and histopatology. AJR Am J Roentgenol 175:1253–1258
Rhim H, Dodd GD (1999) Radiofrequency thermal ablation of liver tumors. J Clin Ultrasound 27:221–229
Carrafiello G, Lagana D, Ianniello A et al (2007) Bleeding after percutaneous radiofrequency ablation: successful treatment with transcatheter embolization. Eur J Radiol 61:351–355
Lencioni R, Cioni D, Crocetti L et al (2005) Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 234:961–967
Livraghi T, Goldberg SN, Lazzaroni S et al (1999) Small hepatocellular carcinoma; treatment with radiofrequency ablation versus ethanol injection. Radiology 210:655–661
Livraghi T, Solbiati L, Meloni MF et al (2003) Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–451
Padhani AR, Ollivier L (2001) The RECIST (response evaluation criteria in solid tumors) criteria: implications for diagnostic radiologists. Br J Radiol 74:983–986
Anderson GS, Brinkmann F, Soulen MC et al (2003) FDG positron emission tomography in the surveillance of hepatic tumors treated with radiofrequency ablation. Clin Nucl Med 28:192–197
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Laganà, D., Carrafiello, G., Mangini, M. et al. Hepatic radiofrequency under CT-fluoroscopy guidance. Radiol med 113, 87–100 (2008). https://doi.org/10.1007/s11547-008-0224-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-008-0224-2