Abstract
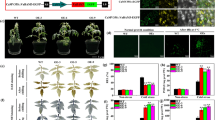
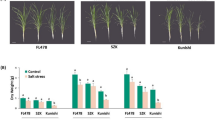
Drought severely affects potato yield and quality. The overexpression of dehydration-responsive element binding proteins/C-repeat-binding factor (DREB1A/CBF3) was previously reported to significantly increase the drought tolerance in transgenic potato (Solanum tuberosum L.), but its molecular mechanism is poorly understood. In the present study, potato cultivar Longshu No. 3 (NT) and its derived transgenic plants (T) with the Arabidopsis DREB1A gene driven by stress-inducible promoter rd29A were used as materials to study drought-stress responses of AtDREB1A in transgenic potato. The results showed, under drought stress, that the AtDREB1A gene was overexpressed in T and T presented a healthier phenotype, higher biomass, higher content of proline and lower content of malondialdehyde than the control NT, indicating that AtDREB1A overexpression improved potato drought tolerance. As the main organ of absorbing and transporting water and nutrients in soil, roots are the first to feel the stress of drought stress. Transcriptome analysis of roots showed that compared with control NT, a total of 533 annotated genes with at least two-fold changes were found to be differentially expressed in transgenic potato roots, comprising 262 up-regulated and 271 down-regulated genes. Among them, the expression of a large number of genes related to abscisic acid metabolism, receptor-like protein kinases, cytochrome P450, the glycosyl hydrolase family, peroxidase, F-box proteins and the heat shock protein family changed greatly, indicating that these genes were responsive to AtDREB1A expression and play important roles in improving drought tolerance of AtDREB1A transgenic potato. This study lays a foundation for further understanding the regulatory network of AtDREB1A gene in improving drought tolerance in potato.






Similar content being viewed by others
Data Availability
Data of physiological indexes generated or analysed during this study are included in this manuscript, and transcriptome data are available in NCBI GenBank under accession number PRJNA578021.
Code Availability
Not applicable.
References
Arroyo-Herrera A, Figueroa-Yáñez L, Castaño E, Santamaría J, Pereira-Santana A, Espadas-Alcocer J, Sánchez-Teyer F, Espadas-Gil F, Alcaraz LD, López-Gómez R, Sánchez-Calderón L, Rodríguez-Zapata LC (2016) A novel Dreb2-type gene from Carica papaya confers tolerance under abiotic stress. Plant Cell Tissue Organ Cult 125:119–133
Bao G, Zhuo C, Qian C, Xiao T, Guo Z, Lu S (2016) Co-expression of NCED and ALO improves vitamin C level and tolerance to drought and chilling in transgenic tobacco and stylo plants. Plant Biotechnol J 14:206–214
Behnam B, Kikuchi A, Celebi-Toprak F, Kasuga M, Yamaguchi-Shinozaki K, Watanabe KN (2007) Arabidopsis rd29A::DREB1A enhances freezing tolerance in transgenic potato. Plant Cell Rep 26:1275–1282
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300
Chen M, Wang QY, Cheng XG, Xu ZS, Li LC, Ye XG, Xia LQ, Ma YZ (2007) GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem Biophys Res Commun 353:299–305
Choi DW, Rodriguez EM, Close TJ (2002) Barley cbf3 gene identification, expression pattern, and map location. Plant Physiol 129:1781–1787
Chu X, Wang C, Chen X, Lu W, Li H, Wang X, Hao L, Guo X (2015) The cotton WRKY Gene GhWRKY41 positively regulates salt and drought stress tolerance in transgenic Nicotiana benthamiana. PLoS One. https://doi.org/10.1371/journal.pone.0143022
Chunthong K, Pitnjam K, Chakhonkaen S, Sangarwut N, Panyawut N, Wasinanon T, Ukoskit K, Muangprom A (2017) Differential drought responses in F-box gene expression and grain yield between two rice groups with contrasting drought tolerance. J Plant Growth Regul 36:970–982
Cui HT, Wang Z, Zhang TJ, Jiang X, Long RC, Yang QC, Kang JM (2018) Cloning and functional analysis of gene MtCYP450 from Medicago truncatula. China J Grassl 5:1–10
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61:651–679
Dahal K, Tai HH, Creelman A, Bizimungu B (2019) Improving potato stress tolerance and tuber yield under a climate change scenario-a current overview. Front Plant Sci 10:563
Dou HO, Xv KP, Meng QW, Li G, Yang XH (2014) Potato plants ectopically expressing Arabidopsis thaliana CBF3 exhibit enhanced tolerance to high-temperature stress. Plant, Cell and Environ 38:61–72
Gao MJ, Allard G, Byass L, Flanagan AM, Singh J (2002) Regulation and characterization of four CBF transcription factors from Brassica napus. Plant Mol Biol 49:459–471
He CY, Zhang JS, Chen SY (2002) A soybean gene encoding a proline-rich protein is regulated by salicylic acid, an endogenous circadian rhythm and by various stresses. Theor & Appl Genet 104(6–7):1125–1131
Huang B, Liu JY (2006) Cloning and functional analysis of the novel gene GhDBP3 encoding a DRE-binding transcription factor from Gossypium hirsutum. Biochim Biophys Acta 1759:263–269
Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI (2010) Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev 24:1695–1708
Jaglo KR, Kleff S, Amundsen KL, Zhang X, Kaake V, Xhan JZ, Deits T, Thomashow MF (2001) Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol 127:910–917
James VA, Neibaur I, Altpeter F (2008) Stress inducible expression of the DREB1A transcription factor from xeric, Hordeum spontaneum L. in turf and forage grass (Paspalum notatum Flugge) enhances abiotic stress tolerance. Transgenic Res 17:93–104
Jia HF, Chai YM, Li CL, Lu D, Luo JJ, Qin L, Shen YY (2011) Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol 157:188–199
Jia XX, Li YT, Qi EF, Ma S, Hu XY, Wen GH, Wang YH, Li JW, Zhang XH, Wang HM, Wang WT (2016) Overexpression of the Arabidopsis DREB1A gene enhances potato drought-resistance. Russ J Plant Physl 63:523–531
Kasuga M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K (2004) A combination of the Arabidopsis DREB1A gene and stress-inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiol 45(3):346–350
Kim H, Hwang H, Hong JW, Lee YN, Ahn IP, Yoon IS, Yoo SD, Lee S, Lee SC, Kim BG (2012) A rice orthologue of the ABA receptor, OsPYL/RCAR5, is a positive regulator of the ABA signal transduction pathway in seed germination and early seedling growth. J Exp Bot 63:1013–1024
Kizis D, Lumbreras V, Pagés M (2001) Role of AP2/EREBP transcription factors in gene regulation during abiotic stress. FEBS Lett 498:187–189
Kudo M, Kidokoro YT, Mizoi J, Todaka D, Fernie AR, Shinozaki K, Yamaguchi-Shinozaki K (2017) Double overexpression of DREB and PIF transcription factors improves drought stress tolerance and cell elongation in transgenic plants. Plant Biotechnol J 15:458–471
Lee SC, Luan S (2012) ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant, Cell Environ 35:53–60
Leng N, Dawson JA, Thomson JA, Ruotti V, Rissman Ai, Smite BMG, Haag JD, Gould MN, Stewart RM, Kendziorski C (2013) EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics 29(8):1035–1043
Libertini E, Li Y, McQueen-Mason SJ (2004) Phylogenetic analysis of the plant endo-β-1, 4-glucanase gene family. J Mol Evol 58:506–515
Lin YP, Wang Y, Jiang SC, Wang KY, Zhang Y, Zhang MP (2015) Advance in research of plant receptor-like protein kinases. Genomics Appl Biol 2:429–437
Li J (2018) ScCBF1 and StCBF1 have different function in response to freezing, drought and salt stress in potato. Dissertation, Shandong Agricultural University, Shandong, China
Li Y, Liu GB, Gao HW, Sui GZ, Zhao HM, Xie N (2010) A comprehensive evaluation of salt-tolerance and the physiological response of medicago sativa at the seedling stage. Acta Pratac Sin 19:79–86
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain, separate two cellular signal transduction pathways in drought- and low temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods 25:402–408
Mao J, Li WF, Mi BQ, Dawuda MM, Caldero´n-Urrea A, Ma ZH, Zhang YM, Chen BH, (2017a) Different exogenous sugars affect the hormone signal pathway and sugar metabolism in ‘“Red Globe”’ (Vitis vinifera L.) plantlets grown in vitro as shown by transcriptomic analysis. Planta 246:537–552
Mao J, Li WF, Mi BQ, Ma ZH, Dawuda MM, Zuo CW, Zhang YM, Jiang XF, Chen BH (2017b) Transcriptome analysis revealed glucose application affects plant hormone signal transduction pathway in “Red Globe” grape plantlets. Plant Growth Regul 84(1):45–56
Movahedi A, Zhang J, Gao P, Yang Y, Wang L, Yin T, Kadkhodaei S, Ebrahimi M, Zhuge Q (2015) Expression of the chickpea CarNAC3 gene enhances salinity and drought tolerance in transgenic poplars. Plant Cell Tissue Organ Cult 120:141–154
O’Kelly BC (2004) Accurate determination of moisture content of organic soils using the oven drying method. Dry Technol 22:1767–1776
Oh SJ, Song SI, Kim YS, Jang HJ, Kim SY, Kim M, Kim YK, Nahm BH, Kim JK (2005) Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. Plant Physiol 138:341–351
Pellegrineschi A, Reynolds M, Pacheco M, Brito RM, Almeraya R, Yamaguchi-Shinozaki K, Hoisington D (2004) Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome 47:493–500
Qin F, Sakuma Y, Li J, Liu Q, Li YQ, Shinozaki K, Yamaguchi-Shinozaki K (2004) Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol 45:1042–1052
Raghavendra AS, Gonugunta VK, Christmann A, Grill E (2010) ABA perception and signalling. Trends Plant Sci 15:395–401
Santiago J, Dupeux F, Round A, Antoni R, Park SY, Jamin M, Cutler SR, Rodriguez PL, Ma’rquez JA, (2009) The abscisic acid receptor PYR1 in complex with abscisic acid. Nature 462:665–668
Shah SH, Ali S, Qureshi AA, Zia MA, Din JU, Ali GM (2017) Chilling tolerance in three tomato transgenic lines overexpressing CBF3 gene controlled by a stress inducible promoter. Environ Sci Pollut Res 24:18536–18553
Shinozaki K, Yamaguchi-Shinozaki K (2000) Gene expression and signal transduction in water-stress response. Curr Opin Plant Biol 3:217–223
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Thomashow MF (2001) So what’s new in the field of plant cold acclimation? Lots! Plant Physiol 125:89–93
Ton J, Flors V, Mauch-Mani B (2009) The multifaceted role of ABA in disease resistance. Trends Plant Sci 14:310–317
Wang LQ, Li Z, Wen SS, Wang JN, Zhao ST, Lu MZ (2019) WUSCHEL-related homeobox gene PagWOX11/12a responds to drought stress by enhancing root elongation and biomass growth in poplar. J Exp Bot 71(4):1503–1513
Wang P, Ma LL, Li Y, Wang SA, Li LF, Yang RT (2017) Transcriptome analysis reveals sunflower cytochrome P450 CYP93A1 responses to high salinity treatment at the seedling stage. Genes Genom 39:581–591
Wei T, Deng K, Liu D, Gao Y, Liu Y, Yang M, Zhang L, Zheng X, Wang C, Song W, Chen C, Zhang Y (2016) Ectopic expression of DREB transcription factor, AtDREB1A, confers tolerance to drought in transgenic Salvia miltiorrhiza. Plant Cell Physiol 57(8):1593–1609
Wycoff KL, Powell PA, Conzales RA, Corbin DR, Lamb C, Dixon RA (1995) Stress activation of a bean hydroxyproline-rich glycoprotein promoter is superimposed on a pattern of tissue-specific developmental expression. Plant Physiol 109:41–52
Xu KP (2011) Studies on the mechanisms of enhanced chilling tolerance in potato transformed with AtCBF3 gene. Dissertation, Shandong Agricultural University, Shandong, China
Xu H, Gao Y, Wang J (2012) Transcriptomic analysis of rice (Oryza sativa) developing embryos using the RNA-seq technique. PloS One. https://doi.org/10.1371/journal.pone.0030646.t001
Yamaguchi-Shinozaki K, Shinozaki K (1993) The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of rd22, a gene responsive to dehydration stress in Arabidopsis thaliana. Mol Gen Genet 238:17–25
Yang HC (2016) The effect of drought stress influence in the proline metabolism pathways of seedling period of beet. Dissertation, Harbin Institute of Technology, Harbin, China
Yang L, Wu K, Gao P, Liu X (2014) GsLRPK, a novel cold-activated leucine-rich repeat receptor-like protein kinase from Glycine soja, is a positive regulator to cold stress tolerance. Plant Sci 215–216:19–28
Yin C, Peng Y, Zang R, Zhu Y, Li C (2010) Adaptive responses of Populus Kangdingensis to drought stress. Physiol Plant 123(4):445–451
Zeng ZM (2016) Functional characterization of Glyoxalase I and Xyloglucan endotransglycosylase in Rice (Oryza sativa L.). Dissertation, Chongqing University, Chongqing, China
Zhang HY, Xie BT, Wang BQ, Dong SX, Duan WX, Zhang LM (2019) Evaluation of drought tolerance and screening for drought-tolerant indicators in sweetpotato cultivars. Acta Agronom Sin 45(3):101–112
Zhao JS, Ren W, Zhi D, Wang L, Xia GM (2007) Arabidopsis DREB1A/CBF3 bestowed transgenic tall fescue increased tolerance to drought stress. Plant Cell Rep 26:1521–1528
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53:247–273
Funding
This work was supported in part by the National Natural Science Foundation of China (grant numbers 31560412, 31060200 and 31860401), the Key Project of Natural Science Foundation of Gansu Province (21JR7RA728), China Agricultural Research System (grant number CARS-09-P06) and the Science and Technology Support Program of Gansu Academy of Agricultural Sciences (grant numbers 2020GAAS16, 2020GAAS10 and 2020GAAS12).
Author information
Authors and Affiliations
Contributions
XXJ, XCZ and GHW conceived and designed the experiments. XXJ, LS and MS conducted experiments and observed plant growth. XXJ, HW and LS performed RT-qPCR and part of the data analysis. EFQ, HPL and XHZ participated in the preparation of the plant material and part of the data analysis. XXJ, MS, LS and XHZ wrote the manuscript. XCZ, HPL and GHW reviewed the manuscript and part of the data analysis. All of the authors in this study read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to participate
Not applicable.
Consent for Publication
All authors give consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, X., Qi, E., Liu, S. et al. Physiological and Transcriptomic Analysis Reveals Drought-Stress Responses of Arabidopsis DREB1A in Transgenic Potato. Potato Res. 66, 1143–1164 (2023). https://doi.org/10.1007/s11540-023-09619-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-023-09619-7