Abstract
This study determines the ability of an isolated Trichosporon domesticum yeast strain to accumulate intracellular lipids in media with deproteinated potato wastewater (DPW) containing various carbon sources. The yeast strain was isolated from kefir and identified by internal transcribed spacer (ITS) region sequence analysis. The sequence was deposited in GenBank under accession number MH094668, and the strain was deposited in Polish Collection of Microorganisms as T. domesticum PCM 2960. DPW is an inexpensive and valuable source of nitrogen, potassium, phosphorus, and other elements in yeast cultures. DPW supplemented with glucose medium was most effective at stimulating lipid biosynthesis by T. domesticum PCM 2960 and bioreactor incubation resulted in a final lipid yield of 4.8 g L−1. The lipids of the T. domesticum PCM 2960 biomass were characterized by high contents of linoleic acid (Δ9,12C18:2), oleic acid (Δ9C18:1), palmitic acid (C16:0), and α-linolenic acid (Δ9,12,15C18:3). Theoretical calculations for biodiesel properties showed that the methylated esters of lipids from T. domesticum PCM 2960 biomass cannot be used as a biodiesel in diesel engines. Additionally, the ability to produce biofilm as one criterion for pathogenicity was tested. The ability for biofilm formation by the isolated strain was low. This study provides a promising solution for the more economical production of microbial lipids with DPW.
Similar content being viewed by others
Introduction
Microbial oils, known as single cell oils (SCOs), are lipids produced by oleaginous microorganisms including yeast, molds, and microalgae. SCO can be used for nutritional and/or energy purposes. Alongside Candida, Rhodotorula, Yarrowia, Rhodosporidium, Cryptococcus, and Lipomyces, yeast from the Trichosporon genera are identified as being oleaginous (Ageitos et al. 2011). Screenings for oleaginous strains are still being performed (Lamers et al. 2016; Viñarta et al. 2016). To reduce the cost of SCO production, low-cost substrates are essential. The search for an appropriate yeast should be directed toward strains that can grow on wastes generated by the agri-food industry.
It has been estimated that during starch processing, 1000 t of potatoes may produce about 600 m3 of arduous liquid wastes (Bzducha-Wróbel et al. 2015). The production of potato starch in the EU-27 countries exceeds 1.7 million tons per year. According to the latest report, titled “Potato Starch Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2018-2023,” the global potato starch market reached a volume of 3.6 million tons in 2017 and is anticipated to reach a volume of 4.0 million tons by 2023. Therefore, it can be calculated that the production of 1000 t of starch generates approximately 3000 m3 of wastewater (calculation for high 20% starch content in potatoes only). This means the global starch industry produced approximately 10.8 million m3 of potato wastewater in 2017. According to the forecast, this amount may increase to over 12 million m3 by 2023. The thermal-acid coagulation of potato wastewater allows potato protein products to be obtained which are used for animal feed. Despite this procedure, remaining deproteinated potato wastewater (DPW) still appears in industrial wastes. DPW is characterized by a high value of chemical oxygen demand (COD), with values from 20 g O2/L (Lubiewski et al. 2006) to 28.5 g O2/L (Kurcz et al. 2018). It can be utilized by field sprinkling which may however lead to adverse eutrophication of water and soil sealing (Kosiek 1993; Lubiewski et al. 2006) in the natural environment. Therefore, there is a real need for the valorization of troublesome starch industrial wastes. The DPW can be used as a medium component that could replace tap water and is a source of nitrogen for yeast breeding. The scheme for DPW formation and the concept of its management for lipid biosynthesis is shown in Fig. 1. The biomass of fodder yeast cultivated on DPW media can be used also as β(1,3)/(1,6)-glucans (Bzducha-Wróbel et al. 2015) or protein source (Kurcz et al. 2018). Moreover, COD index value reduction during yeast cultivation was observed. For instance, COD value decreased by 74% in the medium containing only potato wastewater during C. utilis ATCC 9950 incubation and over 90% in medium supplemented by glycerol (Kurcz et al. 2018).
Trichosporon is a genus of anamorphic basidiomycetous yeast which is widely distributed in temperate and tropical areas, rotting wood, sludge, soil, mushrooms, plants, leaf-cutting ants, birds, and mammals (Obana et al. 2010). Trichosporon yeasts were isolated from rhizospheres contaminated with heavy metals (Ramos-Garza et al. 2016). The genus Trichosporon is a medically important yeast that includes a number of species causing both deep-seated and superficial infections. It has been shown that the Trichosporon species can be divided into at least four distinct groups: serotypes I, II, III, and I–III (reacting with both factors I and III) (Nishiura et al. 1997; Sugita et al. 1997).
Despite this, many representatives of the genus Trichosporon have great biotechnological potential. Among these, the D-xylose assimilating strain Trichosporon mycotoxinivorans can saccharificate sugarcane bagasse hydrolysate. Additionally, it shows both ethanol-tolerance and thermotolerance (Matos et al. 2012). Extracellular β-glucosidase from Trichosporon asahii exhibits a good ability in hydrolyzing aromatic precursors that remain in young finished wine. It can be used for the enhancement and sensory evaluation of wines (Wang et al. 2012). The T. asahii strain can also effectively convert isoeugenol into vanillin with a high molar yield (Ashengroph and Amini 2017). Phenylalanine ammonia lyase (PAL, EC 4.3.1.24) catalyzes the conversion of phenylalanine to trans-cinnamic acid (Gilbert and Tully 1982). PAL from Trichosporon cutaneum can metabolize both Phe and Tyr, since the His-Gln motif (substrate selectivity switch region) is present and indicates the enzyme’s ability to act on both substrates. The enzyme has an optimum pH in the range of 8.0–8.5 and an optimum temperature of 32 °C and shows no metal dependence on the chloride salts of sodium, potassium, magnesium, or ferrum (Goldson-Barnaby and Scaman 2013). Trichosporon fermentans can produce lipids with various cheap substrates such as rice straw hydrolysates (Huang et al. 2009) and molasses (Zhu et al. 2008; Shen et al. 2015) or nitrogen-rich Jerusalem artichoke hydrolysates (Bao et al. 2018). T. cutaneum produces biomass rich in oils after incubation on steam-exploded and three-stage enzymatic hydrolysis corn stover. An efficient and comprehensive process from corn stover to long-chain α,ω-dicarboxylic acids (DCAs) has been established by employing self-metathesis, capable of producing over long-chain DCAs and alkenes as by-products (Zhao et al. 2017). DCAs are important platform chemicals and building blocks for biodegradable polymers (Ngo et al. 2006). It is also possible to employ n-alkanes as inexpensive substrates for biotransformation into microbial lipids by T. cutaneum (Matakova et al. 2017).
This work determines the ability of the Trichosporon domesticum strain isolated from kefir to accumulate intracellular lipids in media with DPW containing glucose, lactose, and glycerol as carbon sources.
Materials and Methods
Isolation
The yeast strain was isolated from commercially available natural kefir within its shelf life period. Ten grams of a kefir sample was aseptically withdrawn and suspended in 90 mL of physiological saline. A 1 mL culture from this dilution was loaded on Sabouraud dextrose agar with chloramphenicol and incubated at 28 °C for 72 h. Several passages using the streaking technique on Sabouraud dextrose agar and incubation at 28 °C resulted in a pure culture yeast strain. The strain was stored in a freezer (− 80 °C) in glycerol protection. Just before the research, the strain was activated by culturing for 24 h on YPD medium.
Identification by DNA Restriction Analysis Method
After 24-h culture in YPD (yeast extract 10 g L−1, peptone 20 g L−1, glucose 20 g L−1) medium, yeast biomass (2 mL) was centrifuged at 716×g and 4 °C for 10 min. DNA from the yeast biomass was isolated following the protocol described by Gientka et al. (2017). The internal transcribed spacer (ITS) region was amplified from yeast ribosomal DNA by PCR using primers ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) (White et al. 1990). PCR amplification was done as follows: 1 min of 95 °C initial denaturation, followed by 30 cycles of 95 °C for 15 s, 55 °C annealing for 15 s, and elongation at 72 °C for 1 min, using DreamTaq polymerase (Thermo Fisher Scientific, USA). The PCR product was visualized on agarose gel. After a quality check, PCR was purified using Exonuclease I/Shrimp Alkaline Phosphatase Mix (Thermo Fisher Scientific, USA) and sequenced using BigDye Terminator v3.1 Mix on an ABI3730xl instrument in the DNA Sequencing and Oligonucleotide Synthesis Laboratory at the Institute of Biochemistry and Biophysics, Polish Academy of Sciences (IBB PAS, Poland). Sequence reads were assembled into a single contig using Seqman software (DNAStar, USA). The obtained consensus sequence was aligned against the GenBank database using BLAST. The sequence was deposited in GenBank under accession number MH094668.
Biofilm Formation
Biofilm formation was investigated by the method described by Messier et al. (2011), with modifications. A yeast inoculum was added to Luria–Bertani (LB) broth (Oxoid) so that the initial yeast cell number was 105 c.f.u. mL−1. This was then transferred to wells in 100-well flat-bottomed Honeycomb plates and incubated in Bioscreen C (Oy Growth Curves Ab. Ltd., Helsinki, Finland) at 37 °C for 48 h. Then, the wells were washed three times gently with 10 mM phosphate-buffered saline (PBS, pH 7.2) and stained with 100 μL 0.1% crystal violet (Sigma-Aldrich, USA) for 30 min at room temperature (25 °C). Excess crystal violet was removed by washing three times with PBS and suspended in 96% ethanol. The biofilm was quantified by measuring absorbance at wavelength λ = 600 nm. The tests were performed tenfold. The use of light wavelengths has been validated (data not shown), and there were no differences compared to λ = 570 nm. Interpretation of results was performed in accordance with the criteria established by Iturrieta-González et al. (2014).
Media and Culture Conditions
DPW was obtained from the processing line of PEPEES SA (Łomża, Poland) and was sterilized (121 °C/0.1 MPa/20 min) (HiCLAVE HG-80 autoclave, HMC Europe). After centrifugation (3200×g/20 min) (Eppendorf 5810 Centrifuge), the aim was to remove all precipitates formed during sterilization; glucose (50 g L−1), lactose (50 g L−1), or glycerol (50 g L−1) was added. The control media (YP) contained yeast extract 10 g L−1, peptone 20 g L−1 and glucose, lactose, or glycerol (50 g L−1). The initial pH of the media was 5.6 ± 0.1 (0.1 M NaOH), and the entire mixture was sterilized (121 °C/0.1 MPa/20 min).
The inoculating cultures (YPD medium) were incubated for 48 h at 28 °C on a reciprocating shaker (SM-30 Control, Buechler, Germany) at a frequency of 200 cycles/min. Experimental cultures were incubated in flasks of 500 mL volume. The various media were inoculated by 10% (v/v) culture, and the cultures were incubated for 96 h at 28 °C at a frequency of 200 cycles/min (SM-30 Control, Buechler, Germany). Each culture variant was replicated three times.
The experimental work was carried out in a 5-L BioFlo 3000 bioreactor (New Brunswick Scientific Co., Edison, NJ, USA) with a working volume of 3 L. Glucose was used as a carbon source at an initial concentration of 50 g L−1 and diluted with DPW. The agitation rate of the bioreactor was 300 rev min−1, and air supply was maintained at a constant rate of 2 vvm and the growth temperature at 28 °C. Foam formation was controlled by the addition of a silicon antifoaming agent (Sigma 204) to a final concentration of 1 ml L−1.
Biomass Yield, Lipids, and Relative Composition of Fatty Acids
The biomass concentration was determined gravimetrically. Determination of lipid content (% CDW) in the biomass was performed by the method of Bligh and Dyer (1959) which was further modified by Zhang et al. (2011). Volumetric lipid efficiency was also calculated, and the mean quantity of lipids produced by 1 L of the medium was assessed.
Determination of fatty acid methyl esters was conducted using a gas chromatography technique coupled with a flame ionization detector (GC-FID TRACE 1300, Thermo Scientific) after prior acid methylation to methyl esters. Methylation and chromatographic procedures were conducted according to Gientka et al. (2017), and the identification of methyl esters was carried out on the basis of retention times of standards present in the mixture (GLC 461 Nu-Chek Prep., Inc., USA).
Calculation of Biodiesel Properties
The unsaturation degree (UD) was theoretically estimated based on the following equation: UD = (1%MU + 2%DU + 3%TU)/100, where %MU is the percentage of weight of the mono-unsaturated methyl esters, %DU is the percentage of weight of the di-unsaturated methyl esters, and %TU is the percentage of weight of the tri-unsaturated methyl esters (Pinzi et al. 2011). The cetane number (CN) was estimated by CN = ΣXMECNME, where XME is the weight percentage of each methyl ester and CNME is the cetane number of individual methyl esters (Ramos et al. 2009). The equation length of chain (LC) = R(nCncn), where nCn is the number of carbon atoms of each fatty acid and cn is the percentage of the weight of each methyl ester containing this fatty acid (Pinzi et al. 2011), describes the LC of the biodiesel. The low caloric value (LCV) was estimated by LCV = 29,385.4 + 486.866 LC − 387.766 UD (kJ kg−1) and viscosity (μ) by μ = − 1.8327 + 0.209794 LC + 0.738911 UD + 0.0166791 LC2 − 0.16336 LC UD + 0.335547 UD2 (mm2 s−1). Flash point (FP) was estimated by FP = 1008.48 − 136.166 LC + 142.578 UD + 5.14811 LC2 − 10.6906 LC UD + 9.26352 UD2, also according to Pinzi et al. (2011).
Statistical Analysis of the Results
All values are means of three separate experiments. The results obtained were statistically analyzed by analysis of variance (ANOVA) using the STATISTICA V.13.1 program (StatSoftPolska Sp. z o.o., Kraków, Poland). Significance of differences between means was determined using Tukey’s test at α = 0.05 level of significance.
Results
Identification
Most species of yeasts can be directly identified using ITS region sequence analysis, and these sequence data have been submitted to the GenBank databases under accession number MH094668. ITS region analyses confirmed that the yeast strain isolate was a T. domesticum. The origin contained 479 bp (Table 1), which are 100% identical to GeneBank deposited sequences of T. domesticum CBS 8280, syn. Apiotrichum domesticum (Scorzetti et al. 2002; Vu et al. 2016), and T. domesticum CMCC O.2 strains. The application of IGS sequence analysis to the identification of species of Trichosporon proved very successful for Sugita et al. (2002). The isolated strain was deposited in Polish Collection of Microorganisms as T. domesticum PCM 2960.
Biofilm Formation
LB medium used in the experiment concerning the ability to form biofilms contained yeast extract 50 g L−1, peptone 10 g L−1 and NaCl 10 g L−1. The growth of the isolated strain in the LB medium was abundant (OD600 1.663 ± 0.132). The biofilm formation ability of T. domesticum PCM 2960, as absorbance readings, was 0.630 ± 0.035 (data not shown). That level of biofilm formation by a strain is substantially low. Iturrieta-González et al. (2014) sought to correlate the capability of strains to biofilm production based on the total range of crystal violet quantifications. Their study showed that this capability was exhibited by over 60 pathogenic isolates of Trichosporon. The authors categorized yeast producers as follows: low capability for biofilm production (A570 < 1), medium (A570 ≥ 1.01 to 2.499) or high (A570 ≥ 2.5).
Biomass Yield and Lipid Content
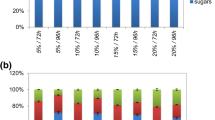
The most preferred source of carbon for biomass yield and the synthesis of lipids in YP media was glucose. On the 3rd day, lipid content was 15% CDW and over the next 24 h this increased to over 27% CDW (Fig. 2). The lipid content (over 20% of dry matter) qualifies the isolated T. domesticum PCM 2960 strain as being oleaginous. The use of glycerol in YP media resulted in lower final biomass yield and lower lipid content (20.5% CDW). Since biomass yield and lipid content were the highest after incubation in medium with glucose, the lipid yield in this case was significantly the highest and exceeded 5 g L−1.
The T. domesticum PCM 2960 strain was able to grow in media based on DPW (Fig. 3). In flask culture, the biomass yield after DPW incubation was lower compared to YP media. The growth of the strain was most effectively promoted by the presence of glycerol, and the biomass yield reached over 11 g L−1 after 96 h of cultivation. The highest content of lipids (almost 28% CDW), resulting in a 2.75 g L−1 lipid yield, was found after culturing with glucose. Despite having the largest biomass yield, glycerol did not have a positive effect on lipid biosynthesis and a low content of lipids (about 20% CDW) was observed. Consequently, lipid yield was significantly lower: 2.38 g L−1.
Bioreactor cultivation with DPW supplemented with glucose resulted in a significant increase in the biomass yield and an increase in the lipid content (Table 2). The final content of lipids was 33% CDW, and as a consequence, lipid yield exceeded 4.8 g L−1.
The lipids of T. domesticum PCM 2960 biomass were characterized by a high content of linoleic acid (Δ9,12C18:2), oleic acid (Δ9C18:1), palmitic acid (C16:0), and α-linolenic acid (Δ9,12,15C18:3) (Table 3). During the period of culturing, an increase in the C16:0, C17:0, C18:0, and C18:2 ratios was observed. Compared to the flask cultures, the lipids from the bioreactor had a higher polyunsaturated fatty acid (PUFA) content and a better ω-6:ω-3 ratio.
Discussion
Identification
Genetic analysis led us to the conclusion that the strain isolated from kefir should be classified as T. domesticum. The yeast species Trichosporon syn. Apiotrichum is included in the family Trichonosporonaceae, order Trichosporonales, class Tremellomycetes, subphylum Agaricomycotina, and phylum Basidiomycota in the Fungi kingdom (NCBI-Taxonomy). Trichosporonales and Tremellales are currently recognized as being Tremellomycetes based on their phenotypic and phylogenetic properties (Liu et al. 2016). However, according to the Integrated Taxonomic Information System (ITS), Trichosporon genus is included in the order Tremellales. The separation of Trichosporonales from Tremellales still remains a matter of debate (Hibbett et al. 2007; Millanes et al. 2011; Liu et al. 2016).
The occurrence of T. domesticum yeast in kefir samples had not thus far been reported. To date, Trichosporon strains have been found in food products several times. Traditional fermented Mongolian cow’s milk features a wide range of yeast species, including the Trichosporon gracile strain (Bai et al. 2010). T. domesticum and T. lactis have been found in bryndza cheese made from raw sheep’s milk with no special starter culture (Laurenčík et al. 2008; Pangallo et al. 2014). Bryndza cheese is a traditional soft spreadable dairy product in Slovakia and is similar to those produced in surrounding countries, such as Poland, Czech Republic, or Ukraine. The yeast T. domesticum is also present in the ripening ewe’s milk cheeses (commercial semi-hard cheeses produced in the spring season with raw milk and bacterial starters) produced in a small traditional dairy in Mediterranean Spain (Padilla et al. 2014). Trichosporon gracile and T. ovoides have been isolated from artisanal cheese (white pickled Serbian and fresh soft Croatian cheese), while T. quercuum has also been isolated from white pickled Serbian cheese. Authors have indicated that these artisanal cheeses are produced in poor hygienic conditions (Golić et al. 2013). T. asahii is present during chhurpi production—a traditional cheese prepared from buttermilk and popular in northeastern India. The absence of these yeasts in prepared (finished) products has been attributed to the heating step during chhurpi production (Rai et al. 2016). It seems reasonable to note that dairy products are a good environment for growth of Trichosporon strains. This confirms our successful isolation of a yeast strain representing Trichosporon genus from natural kefir.
Fermented vegetable-based food can also be a source of Trichosporon species. T. asahii are the main yeast species in mashita (Ongol and Asano 2009). Mashita is a traditional fermented butter-like product used for Ghee production in western Uganda. T. asahii strain with killer activity has been isolated from tempeh (Malaysia) (Lim and Tay 2011). T. asahii has also been found in fura, which is a spontaneously fermented pearl millet product consumed in West Africa (Pedersen et al. 2012). Fermented pepper is one of the traditional Chinese fermented vegetables that can also contain Trichosporon yeast (Zhao et al. 2016).
A Biofilm Formation
Studies addressing virulence factors in Trichosporon spp. have been undertaken by Karashima et al. (2002) and Fonseca et al. (2009), and these have been targeted at an analysis of glucuronoxylomannan (GXM). GXM is a well-described virulence factor of pathogenic yeast species in the Cryptococcus and Trichosporon genus. However, trichosporal and cryptococcal GXMs manifest major structural differences (Fonseca et al. 2009). Cell wall and soluble polysaccharides were isolated from the type strain of T. domesticum. The fractions contained O-acetyl groups, which contributed to their serological reactivity. The polysaccharide has an α-(1.3)-D-mannan backbone with hetero-oligosaccharide side chains consisting of a 2-O-substituted β-D-glucuronic acid residue bound to O-2 of the mannose residue, β-D-xylopyranosyl residues located in the middle of the side chain, and a non-reducing terminal α-L-arabinopyranosyl residue bound to O-4 of xylose. The mannan backbone is O-acetylated at O-6 of the mannose residues (Ichikawa et al. 2001). Protease and phospholipase production and hemolytic activity are other yeast virulence factors (Sun et al. 2012). Biofilm formation is also an important property of pathogenic yeast that helps to cause many types of infections (Messier et al. 2011). Biofilm infections are rather difficult to treat, and they also limit the penetration of antimicrobial drugs or antibodies. Therefore, the ability to produce biofilm can be regarded as a criterion for the pathogenicity of a yeast. The ability of T. domesticum PCM 2960 strain to form biofilm is low. It is lower than the biofilm formation activity of the pathogenic strains of Trichosporon spp. obtained from different patients by Iturrieta-González et al. (2014). Therefore, the assertion seems to be validated that T. domesticum does not meet this criterion of pathogenicity.
Biomass Yield and Lipid Content
Glucose was the source of carbon which most effectively stimulated lipid biosynthesis by T. domesticum PCM 2960. The strain is able to assimilate lactose and glycerol which also means that it has a great potential to aid the biotechnological utilization of whey or crude glycerol. The latter is a waste produced during the production of biodiesel and increases in the amount of this waste is a challenge for the development of this fuel. Undoubtedly, however, crude glycerol could be a low-cost carbon source for lipid biosynthesis. Use of crude glycerol as the sole carbon source has led to the maximum lipid content and lipid yield for T. fermentans CICC 1368 and T. cutaneum AS 2.0571 strains: 32.4%, 5.2 g/L, and 32.2%, 5.6 g/L, respectively (Liu et al. 2017). The maximum content obtained in our study was 19.85% CDW after incubation with DPW and glycerol. Therefore, further optimization of the medium composition and culture conditions should increase the lipid biosynthesis efficiency.
The final content of lipids in T. domesticum PCM 2960 biomass after bioreactor incubation with DPW with glucose was 33% CDW and as a consequence lipid yield exceeded 4.8 g L−1. T. fermentans CICC 1368 showed low lipid accumulation on pure molasses, but enzymatic hydrolysate supplementation of sweet potato vines increased lipid content (Shen et al. 2015). Inulin can be directly utilized by T. cutaneum for microbial lipid fermentation without a hydrolysis step and 4.79 g/L of lipid has been produced from 50 g/L inulin (Wang et al. 2015). The lipid productivity of the T. domesticum strain in DPW medium is similar to that for those cited.
Although the carbon source is a determinant of lipid biosynthesis, nitrogen is also a factor that influences it. By choosing an easily digestible source of nitrogen, a large biomass yield can be expected, and rapid nitrogen utilization from the medium accelerates the start of lipid accumulation. In our previous research, we demonstrated that the use of DPW for the cultivation of Candida and Rhodotorula yeast and for obtaining valuable metabolites, such as β-glucans, proteins, lipids, and carotenoids, is possible. DPW is a good source of available forms of nitrogen, and it is also a source of phosphorus, potassium, magnesium, and other micronutrients necessary for the growth of yeast (Bzducha-Wróbel et al. 2015; Gientka et al. 2017; Kot et al. 2017).
High yeast lipid concentrations and productivity can only be attained if a high yeast cell density is achieved during the growth phase. High yeast cell density can only be achieved in appropriately selected bioreactors. Bioreactor cultivation resulted in a significant increase in biomass yield of T. domesticum PCM 2960 and an increase in the lipid content. It is expected that further work on lipid biosynthesis optimization during bioreactor culturing with the isolated strain will result in increased lipid yield.
A high content of linoleic acid (Δ9,12C18:2), oleic acid (Δ9C18:1), palmitic acid (C16:0), and α-linolenic acid (Δ9,12,15C18:3) was observed in T. domesticum PCM 2960 lipids during incubation with DPW and glucose. However, T. fermentans CICC 1368 lipids produced on molasses-based media contained stearic (C18:0) and linolenic acids (C18:3). Oleic acid (C18:1) was only be detected at low levels in sugar-based media and was not detected after incubation with glycerol (Shen et al. 2015). Three major fatty acids of the same strain of oils obtained from medium with fructose were palmitic acid (16:0), stearic acid (18:0), and oleic acid (18:1), and these three fatty acids accounted for over 95% of the total fatty acids (Bao et al. 2018).
The possibility of using microbial lipids is determined by fatty acid composition. Two dominant applications must be considered. One of these is an approach that can be called nutritional or food technology implementation. Microbiological lipids may be a substitute for valuable plant oils or animal fats, especially rarely occurring fatty acids. The second approach is the possibility to use microbiological lipids for technical purposes. Single cell oils can be a source for diesel engines after esterification reactions.
Among the bioactive components of oils, a key role is played by PUFAs because of their important role in the prevention of metabolic diseases. According to specialist recommendations in the field of human nutrition, the amount of PUFA in the diet should be increased. Among these acids, in addition to linoleic acid (Δ9,12C18:2) and long-chain polyunsaturated fatty acids (LC-PUFA), the following play an especially important role: α-linolenic acid (ALA, Δ9,12,15C18:3) and γ-linolenic acid (Δ6,9,12C18:3), which belong to two biochemically different families, n-3 and n-6, respectively. Recently, particular emphasis has been placed on the important physiological and pro-health roles of n-3 acids, especially in the prevention of diseases of the circulatory system. According to health recommendations (Simopoulos 2011), a favorable ratio of ω-6 to ω-3 for fatty acids from edible oils would be 2:1. The ratio of these fatty acids in the lipids obtained from the biomass of T. domesticum PCM 2960 yeast strain cells after cultivation in the medium DPW with glucose is 7.4:1, which is close to the characteristics of olive oil (9:1).
Fatty acid desaturase activity may be estimated by the calculation of the ratios of desaturase product to the substrate (Fakas et al. 2009). The high C18:1/C18:0 ratios (3.52–3.83) compared with the C16:1/C16:0 ratios (0.03–0.08) during T. domesticum PCM 2960 incubation with DPW may suggest important Δ9 desaturase activity that has a better affinity for fatty acids with 18 carbon atoms than for those with 16 carbon atoms. Makri et al. (2010) and Gientka et al. (2017) demonstrated a high activity of Δ9 desaturase during the exponential phase of yeast growth. The high levels of C18:1/C18:0 in Trichosporon lipids on the final culturing day should be explained by the further multiplication of cells. However, Δ9 desaturase activity decreases with the period of incubation, and an inverse relationship was observed for Δ12 desaturase.
The large content of unsaturated and mono-unsaturated acids in microbial oil mainly determines its biodiesel properties. Based on the fatty acid composition, the obtained fuel can theoretically be calculated. The results are shown in Table 3. The cetane number of the biodiesel should have a minimum value of 54 according to EN 14214 (UE countries) or 47 according to ASTM D6751 (USA), because fuels with low cetane numbers tend to increase gaseous and particulate exhaust emissions (Knothe 2005). The rather low cetane number of the SCO from Trichosporon means that it does not meet the expectations of either standard. The LCV of diesel fuel should be 43 MJ kg−1 (Oliveira and da Silva 2013), and this is another important parameter of a fuel representing the amount of heat transferred to the chamber during combustion and indicates the available energy in a fuel (Demirbas 2008). The flash points (FP) of biodiesel extracted from Trichosporon oils are at more than 122 °C (according to EN 14214 and ASTM D6751), which means the yeast oil is safe to store (Pinzi et al. 2011). The high predicted viscosity of the yeast oil after incubation with DPW medium means that it would meet the expectations of the American standard only. This result shows that the methylated esters of lipids from T. domesticum PCM 2960 yeast cultivated on DPW with glucose cannot be used as a biodiesel in diesel engines.
Conclusion
The results of our experiments confirmed that T. domesticum PCM 2960 isolated from kefir is an oleaginous yeast strain. Research on increasing the lipidogenic potential of the T. domesticum strain leading to the practical use of its oil should be continued. It is possible to focus strategies to enhance lipid productivity including optimization of temperature and oxygenation conditions, as well as genetic engineering, and metabolic engineering toward intracellular lipids enrichment with PUFAs. Our study confirmed the possibility of the application of DPW supplemented by a carbon source in SCO synthesis by T. domesticum PCM 2960 yeast.
Starch processing plants should be interested in simultaneous valorization of DPW and yeast biomass, which could increase their profits by expanding the range of eco-friendly feeding products rich in lipids.
References
Ageitos JM, Vallejo JA, Veiga-Crespo P, Villa TG (2011) Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol 90:1219–1227. https://doi.org/10.1007/s00253-011-3200-z
Ashengroph M, Amini J (2017) Bioconversion of isoeugenol to vanillin and vanillic acid using the resting cells of Trichosporon asahii. 3 Biotech 7(358). https://doi.org/10.1007/s13205-017-0998-9
Bai M, Qing M, Guo Z, Zhang Y, Chen X, Bao Q, Zhang H, Sun T-S (2010) Occurrence and dominance of yeast species in naturally fermented milk from the Tibetan Plateau of China. Can J Microbiol 56(9):707–714. https://doi.org/10.1139/W10-056
Bao R, Wu X, Liu S, Xie T, Yu C, Lin X (2018) Efficient conversion of fructose-based biomass into lipids with Trichosporon fermentans under phosphate-limited conditions. Appl Biochem Biotechnol 184:113–123. https://doi.org/10.1007/s12010-017-2536-y
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bzducha-Wróbel A, Błażejak S, Molenda M, Reczek L (2015) Biosynthesis of β (1,3)/(1,6)-glucans of cell wall of the yeast Candida utilis ATCC 9950 strains in the culture media supplemented with deproteinated potato juice water and glycerol. Eur Food Res Technol 240:1023–1034. https://doi.org/10.1007/s00217-014-2406-6
Demirbas A (2008) Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers Manag 49:125–130. https://doi.org/10.1016/j.enconman.2007.05.002
Fakas S, Makri A, Mavromati M, Tselepi M, Aggelis G (2009) Fatty acid composition in lipid fractions lengthwise the mycelium of Mortierella isabellina and lipid production by solid state fermentation. Bioresour Technol 100:6118–6120. https://doi.org/10.1016/j.biortech.2009.06.015
Fonseca FL, Frases S, Casadevall A, Fischman-Gompertz O, Nimrichter L, Rodrigues ML (2009) Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet Biol 46:496–505. https://doi.org/10.1016/j.fgb.2009.03.003
Gientka I, Kieliszek M, Jermacz K, Błażejak S (2017) Identification and characterization of oleaginous yeast isolated from kefir and its ability to accumulate intracellular fats in deproteinated potato wastewater with different carbon sources. BioMed Res Int 2017:6061042–6061019. https://doi.org/10.1155/2017/6061042
Gilbert HJ, Tully M (1982) Synthesis and degradation of phenylalanine ammonia-lyase of Rhodosporidium toruloides. J Bacteriol 150(2):498–505
Goldson-Barnaby A, Scaman CH (2013) Purification and characterization of phenylalanine ammonia lyase from Trichosporon cutaneum. Enz Res 670702:1–6. https://doi.org/10.1155/2013/670702
Golić N, Čadež N, Terzić-Vidojević A, Šuranská H, Beganović J, Lozo J, Kos B, Šušković J, Raspor P, Topisirović L (2013) Evaluation of lactic acid bacteria and yeast diversity in traditional white pickled and fresh soft cheeses from the mountain regions of Serbia and lowland regions of Croatia. Int J Food Microbiol 166:294–300. https://doi.org/10.1016/j.ijfoodmicro.2013.05.032
Hibbett DA, Binder M, Bischoff JF, et al. (67 authors) (2007) A high-level phylogenetic classification of the fungi. Mycol Res 3:509–547. https://doi.org/10.1016/j.mycres.2007.03.004, 2007
Huang C, Zong MH, Wu H, Liu QP (2009) Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresour Technol 100:4535–4538. https://doi.org/10.1016/j.biortech.2009.04.022
Ichikawa T, Nishikawa A, Wada H, Ikeda R, Shinoda T (2001) Structural studies of the antigen III cell wall polysaccharide of Trichosporon domesticum. Carbohydr Res 330:495–503. https://doi.org/10.1046/j.0014-2956.2001.02438.x
Iturrieta-González IA, Padovan ACB, Bizerra FC, Hahn RC, Colombo AL (2014) Multiple species of Trichosporon produce biofilms highly resistant to triazoles and amphotericin B. PLoS One 9(10):e109553. https://doi.org/10.1371/journal.pone.0109553
Karashima R, Yamakami Y, Yamagata E, Tokimatsu I, Hiramatsu K, Nasu M (2002) Increased release of glucuronoxylomannan antigen and induced phenotypic changes in Trichosporon asahii by repeated passage in mice. J Med Microbiol 51:423–432. https://doi.org/10.1099/0022-1317-51-5-423
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86:1059–1070. https://doi.org/10.1016/j.fuproc.2004.11.002
Kosiek E (1993) Applicability of potato juice in baker’s yeast production. Zeszyty Naukowe Politechniki Łódzkiej Technologia Chemia Spożywcza 648:31–41 [in Polish]
Kot AM, Błażejak S, Kurcz A, Bryś J, Gientka I, Bzducha-Wróbel A, Maliszewska M, Reczek L (2017) Effect of initial pH of medium with potato wastewater and glycerol on protein, lipid and carotenoid biosynthesis by Rhodotorula glutinis. Electron J Biotechnol 27:25–31. https://doi.org/10.1016/j.ejbt.2017.01.007
Kurcz A, Błażejak S, Kot AM, Bzducha-Wróbel A, Kieliszek M (2018) Application of industrial wastes for the production of microbial single-cell protein by fodder yeast Candida utilis. Waste Biomass Valor 9:57–64. https://doi.org/10.1007/s12649-016-9782-z
Lamers D, van Biezen N, Martens D, Peters L, van de Zilver E, van Dreumel JN, Wijffels RH, Lokman C (2016) Selection of oleaginous yeasts for fatty acid production. BMC Biotechnol 16(45). https://doi.org/10.1186/s12896-016-0276-7
Laurenčík M, Sulo P, Sláviková E, Piecková E, Seman M, Ebringer L (2008) The diversity of eukaryotic microbiota in the traditional Slovak sheep cheese—Bryndza. Int J Food Microbiol 127:176–179. https://doi.org/10.1016/j.ijfoodmicro.2008.06.016
Lim SL, Tay ST (2011) Diversity and killer activity of yeasts in Malaysian fermented food samples. Res Note Tropic Biomed 28(2):438–443
Liu X-Z, Wang Q-M, Göker M, Groenewald M, Kachalkin AV, Lumbsch HT, Millanes AM, Wedin M, Yurkov AM, Boekhout T, Bai F-Y (2016) Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol 81:85–147. https://doi.org/10.10162Fj.simyco.2015.12.001
Liu L-P, Hu Y, Lou W-Y, Li N, Wu H, Zong M-H (2017) Use of crude glycerol as sole carbon source for microbial lipid production by oleaginous yeasts. Appl Biochem Biotechnol 182:495–510. https://doi.org/10.1007/s12010-016-2340-0
Lubiewski Z, Śmigielska H, Lewandowicz G, Balcerek W (2006) Charakterystyka odcieku po koagulacji białka pozyskiwanego w toku kampanii krochmalniczej. Zesz Probl Postep Nauk Rol 511:617–626 [in Polish]
Makri A, Fakas S, Aggelis G (2010) Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour Technol 101(7):2351–2358. https://doi.org/10.1016/j.biortech.2009.11.024
Matakova O, Gharwalova L, Zimola M, Rezanka T, Masak J, Kolouchova I (2017) Using odd-alkanes as a carbon source to increase the content of nutritionally important fatty acids in Candida krusei, Trichosporon cutaneum, and Yarrowia lipolytica. Int J Anal Chem Article ID 8195329:9. https://doi.org/10.1155/2017/8195329
Matos ITSR, Cassa-Barbosa LA, Neto PQC, Filho SA (2012) Cultivation of Trichosporon mycotoxinivorans in sugarcane bagasse hemicellulosic hydrolysate. Elec J Biotechnol. https://doi.org/10.2225/vol14-issue1-fulltext-2 RESEARCH ARTICLE
Messier C, Epifano F, Genovese S, Grenier D (2011) Inhibition of Candida albicans biofilm formation and yeast-hyphal transition by 4-hydroxycordoin. Phytomed 18:380–383. https://doi.org/10.1016/j.phymed.2011.01.013
Millanes AM, Diederich P, Ekma S, Wendin M (2011) Phylogeny and character evolution in the jelly fungi (Tremellomycetes, Basidiomycota, Fungi). Mol Phyl Evol 61:12–28. https://doi.org/10.1016/j.ympev.2011.05.014
Ngo HL, Jones K, Foglia TA (2006) Metathesis of unsaturated fatty acids: synthesis of long-chain unsaturatedalpha, omega-dicarboxylic acids. J Am Oil Chem Soc 83:629–634. https://doi.org/10.1007/s11746-006-1249-0
Nishiura Y, Nakagawa-Yoshida K, Suga M, Shinoda T, Guého E, Ando M (1997) Assignment and serotyping of Trichosporon species: the causative agents of summer-type hypersensitivity pneumonitis. J Med Vet Mycol 35:45–52
Obana Y, Sano M, Jike T, Homma T, Nemoto N (2010) Differential diagnosis of trichosporonosis using conventional histopathological stains and electron microscopy. Histopathol 56(3):372–383. https://doi.org/10.1111/j.1365-2559.2010.03477.x
Oliveira LE, Da Silva MLCP (2013) Comparative study of calorific value of rapeseed, soybean, jatrophacurcas and crambe biodiesel. RE&PQJ 1(11):679–682. https://doi.org/10.24084/repqj11.411
Ongol MP, Asano K (2009) Main microorganisms involved in the fermentation of Ugandan ghee. Int J Food Microbiol 133(3):286–291. https://doi.org/10.1016/j.ijfoodmicro.2009.06.003
Padilla B, Manzanares P, Belloch C (2014) Yeast species and genetic heterogeneity within Debaryomyces hansenii along the ripening process of traditional ewes’ and goats’ cheeses. Food Microbiol 38:160–166. https://doi.org/10.1016/j.fm.2013.09.002
Pangallo D, Šaková N, Koreňová J, Puškárová A, Kraková L, Valík L, Kuchta T (2014) Microbial diversity and dynamics during the production of May bryndza cheese. Int J Food Microbiol 170:38–43. https://doi.org/10.1016/j.ijfoodmicro.2013.10.015
Pedersen LL, Owusu-Kwarteng J, Thorsen L, Jespersen L (2012) Biodiversity and probiotic potential of yeasts isolated from Fura, a West African spontaneously fermented cereal. Int J Food Microbiol 159:144–151. https://doi.org/10.1111/jam.12875
Pinzi S, Leiva D, Arzamendi G, Gandia LM, Dorado MP (2011) Multiple response optimization of vegetable oils fatty acid composition to improve biodiesel physical properties. Bioresour Technol 102:7280–7288. https://doi.org/10.1016/j.biortech.2011.05.005
Potato starch market: global industry trends, share, size, growth, opportunity and forecast 2018-2023. Research and Markets. The world’s largest market research store. https://www.researchandmarkets.com/reports/4535068/potato-starch-market-global-industry-trends. Accessed 5 November 2018
Rai AK, Kumari R, Sanjukta S, Sahoo D (2016) Production of bioactive protein hydrolysate using the yeasts isolated from soft chhurpi. Bioresour Technol 219:239–245. https://doi.org/10.1016/j.biortech.2016.07.129
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez A (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100(1):261–268. https://doi.org/10.1016/j.biortech.2008.06.039
Ramos-Garza J, Bustamante-Brito R, de Paz GA, Medina-Canales GM, Vásquez-Murrieta MS, Wang ET, Rodríguez-Tovar AV (2016) Isolation and characterization of yeasts associated with plants growing in heavy-metal- and arsenic-contaminated soils. Can J Microbiol 62(4):307–319. https://doi.org/10.1139/cjm-2015-0226
Scorzetti G, Fell JW, Fonseca A, Statzell-Tallman A (2002) Systematics of basidiomycetous yeasts: a comparison of large subunit D1/D2 and internal transcribed spacer rDNA regions. FEMS Yeast Res 2:495–517. https://doi.org/10.1111/j.1567-1364.2002.tb00117.x
Shen Q, Lin H, Wang Q, Fan X, Yang Y, Zhao Y (2015) Sweetpotato vines hydrolysate promotes single cell oils production of Trichosporon fermentans in high-density molasses fermentation. Bioresour Technol 176:249–256. https://doi.org/10.1016/j.biortech.2014.11.045
Simopoulos AP (2011) Evolutionary aspects of diet: the omega-6/ omega-3 ratio and the brain. Mol Neurobiol 44(2):203–215. https://doi.org/10.1007/s12035-010-8162-0
Sugita T, Makimura K, Nishikawa A, Uchida K, Yamaguchi H, Shinoda T (1997) Identification of medically relevant Trichosporon species based on sequences of internal transcribed spacer regions and construction of a database for Trichosporon identification. Microbiol Immunol 41:571–573
Sugita T, Nakajima M, Ikeda R, Matsushima T, Shinoda T (2002) Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J Clin Microbiol 40(5):1826–1830. https://doi.org/10.1128/JCM.40.5.1826-1830.2002
Sun W, Su J, Xu S, Yan D (2012) Trichosporon asahii causing nosocomial urinary tract infections in intensive care unit patients: genotypes, virulence factors and antifungal susceptibility testing. J Med Microbiol 61:1750–1757. https://doi.org/10.1099/jmm.0.049817-0
Viñarta SC, Angelicola MV, Barros JM, Fernández PM, Mac Cormak W, Aybar MJ, de Figueroa LIC (2016) Oleaginous yeasts from Antarctica: screening and preliminary approach on lipid accumulation. J Basic Microbiol 56:360–1368. https://doi.org/10.1002/jobm.201600099
Vu D, Groenewald M, Szöke S, Cardinali G, Eberhardt U, Stielow B, de Vries M, Verkleij GJM, Crous PW, Boekhout T, Robert V (2016) DNA barcoding analysis of more than 9 000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud Mycol 85:91–105. https://doi.org/10.1016/j.simyco.2016.11.007
Wang Y, Xu Y, Li J (2012) A novel extracellular β-glucosidase from Trichosporon asahii: yield prediction, evaluation and application for aroma enhancement of Cabernet Sauvignon. J Food Sci 77(8):M505–M515. https://doi.org/10.1111/j.1750-3841.2012.02705.x
Wang J, Zhang H, Bao J (2015) Characterization of inulin hydrolyzing enzyme(s) in oleaginous yeast Trichosporon cutaneum in consolidated bioprocessing of microbial lipid fermentation. Appl Biochem Biotechnol 177:1083–1098. https://doi.org/10.1007/s12010-015-1798-5
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press Inc, New York, pp 315–322
Zhang G, French WT, Hernandez R, Alley E (2011) Effect of furfural and acetic acid on grown and lipid production from glucose and xylose by Rhodotorula glutinis. Biomass Bioeng 35:734–740. https://doi.org/10.1016/j.biombioe.2010.10.009
Zhao C, Fang H, Chen S (2017) Single cell oil production by Trichosporon cutaneum from steam-exploded corn stover and its upgradation for production of long-chain α,ω-dicarboxylic acids. Biotechnol Biofuels 10(202). https://doi.org/10.1186/s13068-017-0889-7
Zhao L, Li Y, Jiang L, Deng F (2016) Determination of fungal community diversity in fresh and traditional Chinese fermented pepper by pyrosequencing. FEMS Microbiol Lett 363:fnw273. https://doi.org/10.1093/femsle/fnw273
Zhu LY, Zong MH, Wu H (2008) Efficient lipid production with Trichosporon fermentans and its use for biodiesel preparation. Bioresour Technol 99:7881–7885. https://doi.org/10.1016/j.biortech.2008.02.033
Acknowledgments
The authors would like to acknowledge Maria Stańkowska at PEPEES S.A. in Łomża (Mazovia Voivodeship, Poland) for providing the deproteinated potato wastewater
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gientka, I., Aleksandrzak-Piekarczyk, T., Bzducha-Wróbel, A. et al. Deproteinated Potato Wastewater as a Sustainable Nitrogen Source in Trichosporon domesticum Yeast Lipids Biosynthesis—a Concept of Valorization of Wastewater from Starch Industry. Potato Res. 62, 221–237 (2019). https://doi.org/10.1007/s11540-018-9408-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-018-9408-x