Abstract
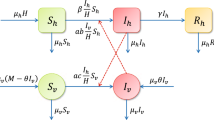
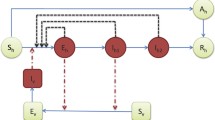
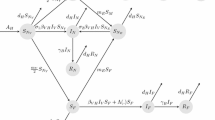
The recent Zika virus outbreak has been spreading rapidly all over the world, expanding its traditionally geographical affected regions, making it a global public health hazard and endangering millions of people. One unique property of the Zika virus compared to most vector-borne diseases is the fact that the virus is transmitted both by mosquitoes and by direct sexual contact. In the present manuscript, we formulate and analyze five mathematical compartmental models of Zika transmission. We model both transmission routes (i.e., vector-borne and sexual transmission). In order to make the model more realistic, heterogeneity in the sexual transmission is modeled in several ways. We fitted the five different models to data, inferred the parameters and selected the most appropriated model, which describes the Zika outbreak in Columbia. For all the models, we estimate the reproduction numbers, namely direct (sexual) transmission, vector transmission and the basic reproduction number \((R_0)\). The analysis revealed that the sexual transmission contribution to \(R_0\) is highest [15.36% (95% CI 12.83–17.4)] for the model which stratifies each gender to high-risk and low-risk individuals in their sexual behavior. For this model, the estimated \(R_0\) is 1.89 (95% CI 1.21–2.13), the direct transmission reproduction number is 0.42 (95% CI 0.29–0.64), and the vector transmission reproduction number is 1.51 (95% CI 1.23–1.87). The sensitivity analysis demonstrated that the value of \(R_0\) depends on three controllable parameters: the biting rate, the sexual transmission rate and the average ratio of mosquito to human.





Similar content being viewed by others
References
Abate A, Tiwari A, Sastry S (2009) Box invariance in biologically-inspired dynamical systems. Automatica 45(7):1601–1610
Agusto FB, Bewick S, Fagan WF (2017a) Mathematical model for Zika virus dynamics with sexual transmission route. Ecol Complex 29:61–81
Agusto FB, Bewick S, Fagan WF (2017b) Mathematical model of Zika virus with vertical transmission. Infect Dis Model 2(2):244–267
Akaike H (1998) Information theory and an extension of the maximum likelihood principle. In: Selected papers of Hirotugu Akaike. Springer, pp 199–213
Allard A, Althouse BM, Hébert-Dufresne L, Scarpino SV (2017a) The risk of sustained sexual transmission of Zika is underestimated. PLoS Pathog 13(9):e1006633
Allard A, Althouse BM, Scarpino SV, Hébert-Dufresne L (2017b) Asymmetric percolation drives a double transition in sexual contact networks. Proc Natl Acad Sci 114(34):8969–8973
Anderson RM, May RM, Anderson B (1992) Infectious diseases of humans: dynamics and control, vol 28. Wiley, Hoboken
Arsuaga M, Bujalance SG, Díaz-Menéndez M, Vázquez A, Arribas JR (2016) Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect Dis 16(10):1107
Atkinson B, Hearn P, Afrough B, Lumley S, Carter D, Aarons EJ, Simpson AJ, Brooks TJ, Hewson R (2016) Detection of Zika virus in semen. Emerg Infect Dis 22(5):940
Brauer F, Castillo-Chavez C, Mubayi A, Towers S (2016) Some models for epidemics of vector-transmitted diseases. Infect Dis Model 1(1):79–87
Calvet G, Aguiar RS, Melo ASO, Sampaio SA, de Filippis I, Fabri A, Araujo ESM, de Sequeira PC, de Mendonça MCL, de Oliveira L et al (2016) Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis 16(6):653–660. https://doi.org/10.1016/S1473-3099(16)00095-5
Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P (2016) Guillain-Barré syndrome outbreak associated with Zika virus infection in French polynesia: a case-control study. Lancet 387(10027):1531–1539
Cao-Lormeau V-M, Musso D (2014) Emerging arboviruses in the pacific. Lancet 384(9954):1571–1572
Castillo-Chavez C, Feng Z, Huang W (2002) On the computation of ro and its role on. Mathematical approaches for emerging and reemerging infectious diseases: an introduction 1:229
Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C et al (2016) Association between Zika virus and microcephaly in french polynesia, 2013–15: a retrospective study. Lancet 387(10033):2125–2132
Coelho FC, Durovni B, Saraceni V, Lemos C, Codeco CT, Camargo S, Carvalho LMD, Bastos L, Arduini D, Villela DAM et al (2016) Higher incidence of zika in adult women than adult men in rio de janeiro suggests a significant contribution of sexual transmission from men to women. Int J Infect Dis 51:128–132
Countrymeters. Colombia population clock. http://countrymeters.info/en/Colombia. Accessed 02 Nov 2016
de Castro Medeiros LC, Castilho CAR, Braga C, de Souza WV, Regis L (2011) Modeling the dynamic transmission of dengue fever: investigating disease persistence. PLoS Negl Trop Dis 5(1):e942
Dick GWA, Kitchen SF, Haddow AJ (1952) Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg 46(5):509–520
Diekmann O, Heesterbeek JAP (2000) Mathematical epidemiology of infectious diseases: model building, analysis and interpretation, vol 5. Wiley, Hoboken
Diekmann O, Heesterbeek JAP, Metz JAJ (1990) On the definition and the computation of the basic reproduction ratio r0 in models for infectious diseases in heterogeneous populations. J Math Biol 28(4):365–382
Duffy MR, Tai-Ho Chen W, Hancock T, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C et al (2009) Zika virus outbreak on yap island, federated states of micronesia. N Engl J Med 360(24):2536–2543
Fauci AS, Morens DM (2016) Zika virus in the Americas yet another arbovirus threat. N Engl J Med 374(7):601–604
Foy BD, Kobylinski KC, Foy JLC, Blitvich BJ, da Rosa AT, Haddow AD, Lanciotti RS, Tesh RB (2011) Probable non-vector-borne transmission of Zika virus, colorado. USA. Emerg Infect Dis 17(5):880–2
Gao D, Lou Y, He D, Porco TC, Kuang Y, Chowell G, Ruan S (2016) Prevention and control of Zika fever as a mosquito-borne and sexually transmitted disease. Sci Rep 6:28070
Gelman A, Carlin JB, Stern HS, Rubin DB (2014) Bayesian data analysis, vol 2. CRC Press, Boca Raton
Gourinat A-C, OConnor O, Calvez E, Goarant C, Dupont-Rouzeyrol M (2015) Detection of Zika virus in urine. Emerg Infect Dis 21(1):84–6
Haario H, Laine M, Mira A, Saksman E (2006) Dram: efficient adaptive MCMC. Stat Comput 16(4):339–354
Haddow AJ, Williams MC, Woodall JP, Simpson DIH, Goma LKH (1964) Twelve isolations of Zika virus from aedes (stegomyia) Africanus (theobald) taken in and above a Uganda forest. Bull World Health Org 31(1):57
Hayes EB et al (2009) Zika virus outside Africa. Emerg Infect Dis 15(9):1347–50
Hills Susan L (2016) Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission continental United States, 2016. MMWR Morb Mortal Wkly Rep 65:215–216
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19(2):101–108
Keeling MJ, Rohani P (2008) Modeling infectious diseases in humans and animals. Princeton University Press, Princeton
Kucharski AJ, Funk S, Eggo RM, Mallet H-P, Edmunds WJ, Nilles EJ (2016) Transmission dynamics of zika virus in island populations: a modelling analysis of the 2013–14 French polynesia outbreak. PLoS Negl Trop Dis 10(5):e0004726
Macnamara FN (1954) Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 48(2):139–145
Marino S, Hogue IB, Ray CJ, Kirschner DE (2008) A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol 254(1):178–196
McKay MD, Beckman RJ, Conover WJ (1979) Comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 21(2):239–245
Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, Kolenc M, Rus KR, Vipotnik TV, Vodušek VF et al (2016) Zika virus associated with microcephaly. N Engl J Med 374(10):951–958
Musso D, Nilles EJ, Cao-Lormeau V-M (2014) Rapid spread of emerging Zika virus in the pacific area. Clin Microbiol Infect 20(10):O595–O596
Musso D, Roche C, Nhan T-X, Robin E, Teissier A, Cao-Lormeau V-M (2015a) Detection of Zika virus in saliva. J Clin Virol 68:53–55
Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau V-M et al (2015b) Potential sexual transmission of Zika virus. Emerg Infect Dis 21(2):359–61
Pan American Health Organization (PAHO) (2016) Zika virus infection. http://www.paho.org/hq/index.php?option=com_content&view=article&id=11585&Itemid=41688&lang= en. Accessed on 16 May 2016
Pan American Health Organization/World Health Organization (2016) Zika—Epidemiological Report Colombia, Oct 2016. PAHO/WHO, Washington, DC. Accessed Oct 2016
Pan American Health Organization WHO, Regional Office for the Americas (2016) Zika cases and congenital syndrome associated with Zika virus reported by countries and territories in the Americas, 2015–2016, cumulative cases. PAHO, WHO, Washington, DC. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=36621&lang=en. Accessed on 20 Oct 2016
Ross R (1916) An application of the theory of probabilities to the study of a priori pathometry. Part I. Proc R Soc Lond Ser A 92(638):204–230
Sardar T, Sasmal SK, Chattopadhyay J (2016) Estimating dengue type reproduction numbers for two provinces of Sri Lanka during the period 2013–14. Virulence 7(2):187–200
Schwarz G et al (1978) Estimating the dimension of a model. Ann Stat 6(2):461–464
Shapshak P, Sinnott JT, Somboonwit C, Kuhn J (2015) Global virology I–identifying and investigating viral diseases. Springer, Berlin
Smith DL, Dushoff J, McKenzie FE (2004) The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol 2(11):e368
Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A (2002) Bayesian measures of model complexity and fit. J R Stat Soc Ser B (Stat Methodol) 64(4):583–639
Towers S, Brauer F, Castillo-Chavez C, Falconar AKI, Mubayi A, Romero-Vivas CME (2016) Estimate of the reproduction number of the 2015 Zika virus outbreak in Barranquilla, Colombia, and estimation of the relative role of sexual transmission. Epidemics 17:50–55
Van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180(1):29–48
World Health Organization (WHO) (2015) Zika virus. Accessed Feb 2015
World Health Organization (WHO) (2016) Zika virus, fact sheet. Online Jan 2016
Zanluca C, de Melo VCA, Mosimann ALP, Santos GIV, Santos CND, Luz K (2015) First report of autochthonous transmission of Zika virus in brazil. Memórias do Instituto Oswaldo Cruz 110(4):569–572
Acknowledgements
We would like to thank the editor and two anonymous reviewers for their helpful comments. SKS is supported by the postdoctoral fellowship from Japan Society for the Promotion of Science (JSPS), Government of Japan. IG is supported by the research fellowship from University Grant Commission (UGC), Government of India. The Funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sourav Kumar Sasmal and Indrajit Ghosh have contributed equally to this work.
Amit Huppert and Joydev Chattopadhyay have contributed equally to this work.
Appendices
Appendix A: Mathematical Analysis for the Model (2)
1.1 Positivity and Boundedness of Solutions for the Model (2)
Lemma
The closed and bounded set
is positively invariant and attracting with respect to the Model (2).
Proof
The system (2) can be written as
where \(X = \left( S_\mathrm{m}, S_\mathrm{f}, I_\mathrm{m}, I_\mathrm{np}, I_\mathrm{p}, R, S_\mathrm{v}, I_\mathrm{v}\right) ^T\). The matrix A is given by
where \(\lambda _\mathrm{m} = \frac{\beta _1\theta _1I_\mathrm{v}}{N_\mathrm{h}} + \frac{\beta _2\left( I_\mathrm{np}+I_\mathrm{p}\right) }{\rho N_\mathrm{h}}\), \(\lambda _\mathrm{f} = \frac{\beta _1\theta _1I_\mathrm{v}}{N_\mathrm{h}} + \frac{\beta _2I_\mathrm{m}}{\rho N_\mathrm{h}}\) and \(\lambda _v = \frac{\beta _1\theta _2\left( I_\mathrm{m}+I_\mathrm{np}+I_\mathrm{p}\right) }{N_\mathrm{h}}\).
The vector \(B = \left( \frac{\mu _\mathrm{h}N_\mathrm{h}}{2}, \frac{\mu _\mathrm{h}N_\mathrm{h}}{2}, 0, 0, 0, 0, \mu _\mathrm{v}N_\mathrm{v}, 0\right) ^T\) is positive. Note that A(X) has all off diagonal entries nonnegative, i.e., A(X) is a Metzler matrix, for all \(X\in \mathbb {R}^8_+\). Since \(B\ge 0\) system (2) is positively invariant in \(\mathbb {R}^8_+\) (Abate et al. 2009). Therefore, any trajectory of the system (2) starting from an initial state in \(\mathbb {R}^8_+\) remains trapped forever in \(\mathbb {R}^8_+\).
Adding the first six equations of the Model (2), we get
Therefore, as \(t\rightarrow \infty \), \(0\le N_\mathrm{h}(t) \le N_\mathrm{h}(0)\). Similarly, by adding last two equations of the Model (2), we get as \(t\rightarrow \infty \), \(0\le N_\mathrm{v}(t) \le N_\mathrm{v}(0)\).
Therefore, all mathematically and biologically feasible solutions of the model system (2) enter the region \(\varOmega \), i.e., \(\varOmega \) is attracting. Hence, it is now sufficient to study the dynamical properties of the Model (2) in \(\varOmega \). \(\square \)
1.2 Disease-Free Equilibrium Stability
The Model (2) has the unique disease-free equilibrium \(E_0 =\left( \frac{N_\mathrm{h}}{2}, \frac{N_\mathrm{h}}{2}, 0, 0, 0, 0,\right. \left. N_\mathrm{v}, 0\right) \). Now by Theorem 2 in Van den Driessche and Watmough (2002), the disease-free equilibrium \(E_0\) is locally asymptotically stable if \(R_0 < 1\), but unstable if \(R_0 > 1\). The global stability of the disease-free equilibrium is given in the following theorem.
Theorem
In the region,
the disease-free equilibrium \(E_0 = \left( \frac{N_\mathrm{h}}{2}, \frac{N_\mathrm{h}}{2}, 0, 0, 0, 0, N_\mathrm{v}, 0\right) \) is globally asymptotically stable equilibrium of (2) provided that \(R_0 < 1\).
Proof
The system (2) can be written as
where \(X = \left( S_\mathrm{m}, S_\mathrm{f}, R, S_\mathrm{v}\right) \in \mathbb {R}^4\) (the number of uninfected individuals compartments), \(Z = \left( I_\mathrm{m}, I_\mathrm{np}, I_\mathrm{p}, I_\mathrm{v}\right) \in \mathbb {R}^4\) (the number of infected individuals compartments), and \(E_0 = \left( \frac{N_\mathrm{h}}{2}, \frac{N_\mathrm{h}}{2}, 0, 0, 0, 0, N_\mathrm{v}, 0\right) \) is the disease-free equilibrium of the system (2).
The following two conditions must be met to guarantee global asymptotic stability:
-
1.
For \(\frac{\hbox {d}X}{\hbox {d}t} = F(X,0)\), \(X^*\) is globally asymptotically stable,
-
2.
\(G(X,Z) = AZ - {\widehat{G}}(X,Z)\), \({\widehat{G}}(X,Z) \ge 0\) for \((X, Z) \in \varOmega \),
where \(A = D_z G(X^*,0)\) is an Metzler matrix and \(\varOmega \) is the region where the model makes biological sense. If the system (2) satisfies the above two conditions, then by the theorem given in Castillo-Chavez et al. (2002), the disease-free equilibrium \(E_0\) is globally asymptotically stable when \(R_0 < 1\). Now,
and
It is clear that \({\widehat{G}}(X,Z)\ge 0\) in the region \(\varOmega _1\). Therefore, it is also clear that \(X^* = \left( \frac{N_\mathrm{h}}{2}, \frac{N_\mathrm{h}}{2}, 0, N_\mathrm{v}\right) \) is a globally asymptotically stable equilibrium of the system \(\frac{\hbox {d}X}{\hbox {d}t} = F(X,0)\). Thus, the above theorem holds. \(\square \)
1.3 Existence of Endemic Equilibria
Suppose \(E_* = \left( S_\mathrm{m}^*, S_\mathrm{f}^*, I_\mathrm{m}^*, I_\mathrm{np}^*, I_\mathrm{p}^*, R^*, S_\mathrm{v}^*, I_\mathrm{v}^*\right) \) is an endemic equilibrium of the Model (2), where
Here, x is the positive roots of the following quadratic equation
where
Endemic equilibria exists if
-
Case I:
If \(\varDelta = B^2 - 4C < 0\), no interior equilibrium exists.
-
Case II:
If \(\varDelta = B^2 - 4C = 0\), unique interior equilibrium may exists.
-
Case III:
If \(\varDelta = B^2 - 4C > 0\), two interior equilibrium may exist.
Appendix B: Some Inference Plots
Posterior distribution of different variables and parameters of the Model (1)
Traces of the model variable and parameter values as obtained by the MCMC sampling for 500, 00 iteration numbers, for the Model (1)
Monotone relationship of sensitivity parameters with respect to the total number of infected pregnant women cases per week, for the Model (1), which is the prerequisite before computing the PRCC
Rights and permissions
About this article
Cite this article
Sasmal, S.K., Ghosh, I., Huppert, A. et al. Modeling the Spread of Zika Virus in a Stage-Structured Population: Effect of Sexual Transmission. Bull Math Biol 80, 3038–3067 (2018). https://doi.org/10.1007/s11538-018-0510-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-0510-7