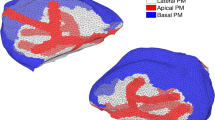
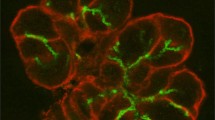
Abstract
We have constructed a spatiotemporal model of \(\hbox {Ca}^{2+}\) dynamics in parotid acinar cells, based on new data about the distribution of inositol trisphophate receptors (IPR). The model is solved numerically on a mesh reconstructed from images of a cluster of parotid acinar cells. In contrast to our earlier model (Sneyd et al. in J Theor Biol 419:383–393. https://doi.org/10.1016/j.jtbi.2016.04.030, 2017b), which cannot generate realistic \(\hbox {Ca}^{2+}\) oscillations with the new data on IPR distribution, our new model reproduces the \(\hbox {Ca}^{2+}\) dynamics observed in parotid acinar cells. This model is then coupled with a fluid secretion model described in detail in a companion paper: A mathematical model of fluid transport in an accurate reconstruction of a parotid acinar cell (Vera-Sigüenza et al. in Bull Math Biol. https://doi.org/10.1007/s11538-018-0534-z, 2018b). Based on the new measurements of IPR distribution, we show that Class I models (where \(\hbox {Ca}^{2+}\) oscillations can occur at constant [\(\hbox {IP}_3\)]) can produce \(\hbox {Ca}^{2+}\) oscillations in parotid acinar cells, whereas Class II models (where [\(\hbox {IP}_3\)] needs to oscillate in order to produce \(\hbox {Ca}^{2+}\) oscillations) are unlikely to do so. In addition, we demonstrate that coupling fluid flow secretion with the \(\hbox {Ca}^{2+}\) signalling model changes the dynamics of the \(\hbox {Ca}^{2+}\) oscillations significantly, which indicates that \(\hbox {Ca}^{2+}\) dynamics and fluid flow cannot be accurately modelled independently. Further, we determine that an active propagation mechanism based on calcium-induced calcium release channels is needed to propagate the \(\hbox {Ca}^{2+}\) wave from the apical region to the basal region of the acinar cell.
















Similar content being viewed by others
References
Boltcheva D, Yvinec M, Boissonnat J (2009) Mesh generation from 3d multi-material images. In: International conference on medical image computing and computer-assisted intervention, pp 283–290. https://doi.org/10.1007/978-3-642-04271-3_35
Bruce JI, Shuttleworth TJ, Giovannucci DR, Yule DI (2002) Phosphorylation of inositol 1, 4, 5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on \(\text{ Ca }^{2+}\) signaling. J Biol Chem 277(2):1340–1348. https://doi.org/10.1074/jbc.M106609200
De Young GW, Keizer J (1992) A single-pool inositol 1, 4, 5-trisphosphate-receptor-based model for agonist-stimulated oscillations in \(\text{ Ca }^{2+}\) concentration. Proc Natl Acad Sci 89(20):9895–9899. https://doi.org/10.1073/pnas.89.20.9895
Desbrun M, Meyer M, Schröder P, Barr A (1999) Implicit fairing of irregular meshes using diffusion and curvature flow. In: Proceedings of the 26th annual conference on computer graphics and interactive techniques SIGGRAPH ’99, pp 317–324. https://doi.org/10.1145/311535.311576
Dickinson GD, Ellefsen KL, Dawson SP, Pearson JE, Parker I (2016) Hindered cytoplasmic diffusion of inositol trisphosphate restricts its cellular range of action. Sci Signal 9(453):ra108
Dupont G, Erneux C (1997) Simulations of the effects of inositol 1, 4, 5-trisphosphate 3-kinase and 5-phosphatase activities on \(\text{ Ca }^{2+}\) oscillations. Cell Calcium 22(5):321–331. https://doi.org/10.1016/S0143-4160(97)90017-8
Dupont G, Goldbeter A (1993) One-pool model for \(\text{ Ca }^{2+}\) oscillations involving \(\text{ Ca }^{2+}\) and inositol 1, 4, 5-trisphosphate as co-agonists for \(\text{ Ca }^{2+}\) release. Cell Calcium 14(4):311–322
Dupont G, Falcke M, Kirk V, Sneyd J (2016) Models of calcium signalling, vol 43. Springer, New York
Friel D (1995) [\(\text{ Ca }^{2+}\)]\(_i\) oscillations in sympathetic neurons: an experimental test of a theoretical model. Biophys J 68(5):1752–1766. https://doi.org/10.1016/S0006-3495(95)80352-8
Gaspers LD, Bartlett PJ, Politi A, Burnett P, Metzger W, Johnston J, Joseph SK, Höfer T, Thomas AP (2014) Hormone-induced calcium oscillations depend on cross-coupling with inositol 1, 4, 5-trisphosphate oscillations. Cell Rep 9(4):1209–1218. https://doi.org/10.1016/j.celrep.2014.10.033
Geuzaine C, Remacle J (2009) Gmsh: a 3-d finite element mesh generator with built-in pre- and post-processing facilities. Int J Numer Methods Eng 79:1309–1331. https://doi.org/10.1002/nme.2579
Harootunian AT, Kao JP, Paranjape S, Tsien RY (1991) Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP\(_3\). Science 251:75–78. https://doi.org/10.1126/science.1986413
Kasai H, Li YX, Miyashita Y (1993) Subcellular distribution of \(\text{ Ca }^{2+}\) release channels underlying \(\text{ Ca }^{2+}\) waves and oscillations in exocrine pancreas. Cell 74(4):669–677. https://doi.org/10.1016/0092-8674(93)90514-Q
Keizer J, Levine L (1996) Ryanodine receptor adaptation and \(\text{ Ca }^{2+}\) (-) induced \(\text{ Ca }^{2+}\) release-dependent \(\text{ Ca }^{2+}\) oscillations. Biophys J 71(6):3477–3487. https://doi.org/10.1016/S0006-3495(96)79543-7
Krane CM, Melvin JE, Nguyen H-V, Richardson L, Towne JE, Doetschman T, Menon AG (2001) Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem 276(26):23,413–23,420. https://doi.org/10.1074/jbc.M008760200
Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S (1997) Polarized expression of \(\text{ Ca }^{2+}\) channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [\(\text{ Ca }^{2+}\)]\(_i\) waves. J Biol Chem 272(25):15,765–15,770. https://doi.org/10.1074/jbc.272.25.15765
Leite MF, Burgstahler AD, Nathanson MH (2002) \(\text{ Ca }^{2+}\) waves require sequential activation of inositol trisphosphate receptors and ryanodine receptors in pancreatic acini. Gastroenterology 122(2):415–427. https://doi.org/10.1053/gast.2002.30982
MacLennan DH, Rice WJ, Green NM (1997) The mechanism of \(\text{ Ca }^{2+}\) transport by sarco (endo) plasmic reticulum \(\text{ Ca }^{2+}\)-ATPases. J Biol Chem 272(46):28,815–28,818. https://doi.org/10.1074/jbc.272.46.28815
Means S, Smith AJ, Shepherd J, Shadid J, Fowler J, Wojcikiewicz RJ, Mazel T, Smith GD, Wilson BS (2006) Reaction diffusion modeling of calcium dynamics with realistic ER geometry. Biophys J 91(2):537–557
Nathanson MH, Fallon MB, Padfield PJ, Maranto AR (1994) Localization of the type 3 inositol 1, 4, 5-trisphosphate receptor in the \(\text{ Ca }^{2+}\) wave trigger zone of pancreatic acinar cells. J Biol Chem 269(7):4693–4696
Nezu A, Morita T, Tanimura A (2015) In vitro and in vivo imaging of intracellular \(\text{ Ca }^{2+}\) responses in salivary gland cells. J Oral Biosci 57(2):69–75. https://doi.org/10.1016/j.job.2015.02.003
Palk L, Sneyd J, Shuttleworth TJ, Yule DI, Crampin EJ (2010) A dynamic model of saliva secretion. J Theor Biol 266(4):625–640. https://doi.org/10.1016/j.jtbi.2010.06.027
Penny CJ, Kilpatrick BS, Han JM, Sneyd J, Patel S (2014) A computational model of lysosome-ER \(\text{ Ca }^{2+}\) microdomains. J Cell Sci 127(13):2934–2943. https://doi.org/10.1242/jcs.149047
Politi A, Gaspers LD, Thomas AP, Höfer T (2006) Models of IP\(_3\) and \(\text{ Ca }^{2+}\) oscillations: frequency encoding and identification of underlying feedbacks. Biophys J 90(9):3120–3133. https://doi.org/10.1529/biophysj.105.072249
Rugis J (2005) Surface curvature maps and Michelangelo’s David. Image Vis Comput N Z 2005:218–222
Rugis J, Klette R (2006a) A scale invariant surface curvature estimator. LNCS Adv Image Video Technol 4319:138–147
Rugis J, Klette R (2006b) Surface registration markers from range scan data. LNCS Comb Image Anal 4040:430–444
Savitzky A, Golay M (1964) Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 36:1627–1639. https://doi.org/10.1021/ac60214a047
Sneyd J, Tsaneva-Atanasova K, Bruce J, Straub S, Giovannucci D, Yule D (2003) A model of calcium waves in pancreatic and parotid acinar cells. Biophys J 85(3):1392–1405. https://doi.org/10.1016/S0006-3495(03)74572-X
Sneyd J, Tsaneva-Atanasova K, Reznikov V, Bai Y, Sanderson M, Yule D (2006) A method for determining the dependence of calcium oscillations on inositol trisphosphate oscillations. Proc Natl Acad Sci USA 103(6):1675–1680. https://doi.org/10.1073/pnas.0506135103
Sneyd J, Han JM, Wang L, Chen J, Yang X, Tanimura A, Sanderson MJ, Kirk V, Yule DI (2017a) On the dynamical structure of calcium oscillations. Proc Natl Acad Sci 14:201614613. https://doi.org/10.1073/pnas.1614613114
Sneyd J, Means S, Zhu D, Rugis J, Won JH, Yule DI (2017b) Modeling calcium waves in an anatomically accurate three-dimensional parotid acinar cell. J Theor Biol 419:383–393. https://doi.org/10.1016/j.jtbi.2016.04.030
Stern MD, Pizarro G, Ríos E (1997) Local control model of excitation–contraction coupling in skeletal muscle. J Gen Physiol 110(4):415–440. https://doi.org/10.1085/jgp.110.4.415
Tanimura A, Morita T, Nezu A, Tojyo Y (2009) Monitoring of IP\(_3\) dynamics during \(\text{ Ca }^{2+}\) oscillations in HSY human parotid cell line with FRET-based IP\(_3\) biosensors. J Med Investig 56:357–361. https://doi.org/10.2152/jmi.56.357 (Supplement)
Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH (1993) Local and global cytosolic \(\text{ Ca }^{2+}\) oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell 74(4):661–668. https://doi.org/10.1016/0092-8674(93)90513-P
Vera-Sigüenza E, Catalàn MA, Peña-Münzenmayer G, Melvin JE, Sneyd J (2018a) A mathematical model supports a key role for Ae4 (Slc4a9) in salivary gland secretion. Bull Math Biol 80(2):255–282. https://doi.org/10.1007/s11538-017-0370-6
Vera-Sigüenza E, Pages N, Rugis J, Yule DI, Sneyd J (2018b) A mathematical model of fluid transport in an accurate reconstruction of parotid acinar cell. Bull Math Biol. https://doi.org/10.1007/s11538-018-0534-z
Tojyo Y, Tanimura A, Matsumoto Y (1997) Imaging of intracellular \(\text{ Ca }^{2+}\) waves induced by muscarinic receptor stimulation in rat parotid acinar cells. Cell Calcium 22(6):455–462. https://doi.org/10.1016/S0143-4160(97)90073-7
Wang IY, Bai Y, Sanderson MJ, Sneyd J (2010) A mathematical analysis of agonist-and KCl-induced \(\text{ Ca }^{2+}\) oscillations in mouse airway smooth muscle cells. Biophys J 98(7):1170–1181. https://doi.org/10.1016/j.bpj.2009.12.4273
Yule DI, Ernst SA, Ohnishi H, Wojcikiewicz RJ (1997) Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1, 4, 5-trisphosphate receptors in pancreatic acinar cells. J Biol Chem 272(14):9093–9098. https://doi.org/10.1074/jbc.272.14.9093
Zhang X, Wen J, Bidasee KR, Besch HR, Rubin RP (1997) Ryanodine receptor expression is associated with intracellular \(\text{ Ca }^{2+}\) release in rat parotid acinar cells. Am J Physiol Cell Physiol 273(4):C1306–C1314. https://doi.org/10.1152/ajpcell.1997.273.4.C1306
Zhang X, Wen J, Bidasee KR, Besch HR, Wojcikiewicz RJH, Lee B, Rubin RP (1999) Ryanodine and inositol trisphosphate receptors are differentially distributed and expressed in rat parotid gland. Biochem J 340(2):519–527. https://doi.org/10.1042/bj3400519
Acknowledgements
This work was supported by the National Institutes of Health grant number RO1DE019245-10 and by the Marsden Fund of the Royal Society of New Zealand. High-performance computing facilities and support were provided by the New Zealand eScience Infrastructure (NeSI) funded jointly by NeSI’s collaborator institutions and through the Ministry of Business, Innovation and Employment’s Research Infrastructure programme. Thanks to NVIDIA Corporation for a K40 GPU grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (mp4 121237 KB)
Appendix: Construction of the Finite-Element Mesh
Appendix: Construction of the Finite-Element Mesh
In this appendix, we describe how we used experimentally measured optical slices to construct a multicellular finite-element mesh, in which the cells have conformal intercellular faces. Our work flow has three sequential steps:
-
1.
Image segmentation
-
2.
Surface triangulation and refinement
-
3.
Volumetric meshing
1.1 Image Segmentation
By visual inspection, we identified and selected a contiguous clump of seven cells within our source data image stack. This clump spanned thirty of the images and covered a maximum of approximately one-quarter of each image. Segmentation was done manually by tracing the outline of each cell in each image with a distinct colour, followed by matched colour flood-fill. One-quarter of original image number 16, along with its segmentation, is shown in Fig. 17.
Note that with our image stack the ratio of stack spacing to pixel resolution is 11.6 (being 0.069 / 0.80). To bring this closer to a one-to-one ratio, we reduced the X and Y dimensions in the segmented image stack by a factor of four using nearest neighbour interpolation (to retain the distinct colouring of each cell) resulting in a pixel spacing of approximately \(0.28\upmu \text {m}\). The reduced images were then combined into a single XYZ TIFF stack for convenience.
1.1.1 Curvature
We also wanted to extract some information from the segmented image stack about the smoothness of the surface of each cell. We chose line curvature as a surface smoothness characteristic indicator (Rugis 2005; Rugis and Klette 2006a, b).
To calculate the curvature, we used the \(1024 \times 1024\) segmented images and extracted pixels associated with the closed curve boundary outline for each cell in each image, then selected the outline containing the maximum number of pixels for each cell as being the closest to a “great arc” slice through that cell. This great arc criterion was based on the fact that only the curvature associated with a great arc of a sphere is equal to the sphere surface mean curvature.
Next, considering that fact that image pixels are all located on a regular rectangular grid (not very useful for calculating actual local curvature!), a smoothed version of each cell outline was created using a 2D Savitzky–Golay (least-squares) fitting filter (Savitzky and Golay 1964). Figure 18 shows a sample cell outline with the original pixel locations as well as the smoothing process result.
Planar line curvature was calculated at each point on the smoothed outlines. We used weighted histograms to visualise the curvature distribution for each of the seven cells. Two of the histograms are shown in Fig. 19. Note that in both cases the curvature distribution is biased towards the positive, as would be expected with any closed curve from the outside using the convention that positive curvature is associated with convex line segments.
To simplify our characterisation of cell surface shape, we chose the weighted standard deviation of the curvatures for each cell as a single valued characterisation of surface smoothness and, in that sense, a signature for the shape of each cell. This signature is indicative (i.e. not unique) because standard deviation is only an unambiguous characterisation given normally distributed data. The weighted curvature standard deviation for each of the seven cells is shown in Table 2.
1.2 Surface Triangulation and Refinement
For the surface triangulation and refinement process, we started with the reduced XYZ TIFF image stack described in the previous section. We treated this reduced stack as a \(256\times 256 \times 31\) solid voxel block within which each voxel is labelled with a colour associated with the cell that it belongs to.
1.2.1 Surface Triangulation
Significant prior work in extracting triangle surface meshes from labelled voxel blocks has been done by Boltcheva et al. (2009), and we used their technique. Sample computer code implementing this technique was found on the Computational Geometry Algorithms Library (CGAL) web site (www.cgal.org). Note that we needed to convert our voxel block data to Inria format (www.inria.fr) before passing it to the CGAL code.
The output from the CGAL code was a multi-domain triangle surface mesh, as shown in Fig. 20, where each of the cell faces are labelled in colour by cell. However, there are two problems with this mesh: there are “steps” as a result of the relatively limited stack resolution and, on close inspection, the surfaces are rather rough (as can be seen more clearly in the cell labelled “no smoothing” in the top of Fig. 21). This lack of surface smoothness has the net effect of increasing the surface area of each cell to a value larger than what it most likely is in the real cells.
1.2.2 Surface Smoothing
To reduce the surface area closer to what it should be, we used a constrained surface smoothing process. Note that the smoothing process should: 1) minimise the surface area of each cell, 2) maintain the volume of each cell, and 3) keep the shared conformal faces between cells as being shared.
A pseudo-code outline for our iterative smoothing process is as follows:

For each cell we used the curvature-flow based smoothing operation described in Desbrun et al. (1999). Note that the cell smoothing process works by slightly moving the position of the surface mesh vertices for each cell in turn (with no new faces added or any faces removed). Therefore, vertices associated with shared faces get moved twice in each pass through the Main Loop, i.e. once for each cell in every adjacent pair of cells. In this sense, the smoothing is a coupled process. With our approach, if the vertices associated with shared faces were allowed to split from each other, the result would be non-coupled as shown in the middle row of Fig. 21, which is not what is required.
Coupled smoothing results after each of 6, 16, 40 and 100 iterations are shown in the bottom row of Fig. 21. The question remains: After how many iterations should we stop? This is where we found the weighted curvature standard deviation values from Table 2 useful as will be described in the next section.
1.2.3 Iteration Termination
Recall from the previous section that reducing cell surface area while at the same time maintaining cell volume was an important consideration. The smoothing step in our process has the effect of reducing surface area as shown for one hundred iterations in the left-hand side of Fig. 22. The greatest rate of surface area reduction occurs within the first ten iterations or so. Cell volume is explicitly restored in our process, and thus remains almost unchanged, as can be seen in the right-hand side of Fig. 22. For further guidance as to when to terminate the iterative process, we looked to surface curvature.
For each cell, in every iteration, we calculated the 2D surface curvature at every mesh vertex. Additionally, for each cell, in every iteration, we calculated the weighted standard deviation of those curvatures to get a single smoothness characteristic value. We then compared this value to the associated target standard deviation in Table 2 and expressed it as a ratio as shown in Fig. 23.
With this information in hand, we decided to terminate the iterative process and accept the results after ten iterations by which time all of the cells had more than dropped below their target characteristic curvature.
For further insight and confirmation of our results, we produced initial and final iteration weighted surface curvature histograms for each of the seven cells. Histograms for two of the cells are shown in Fig. 24. Note that in all cases, as expected, the initial curvature spread was relatively wide and the final curvature distribution narrower, peaking just to the right (positive) side of zero. The final curvature histograms compared favourably to those associated with the image stacks including those shown earlier in Fig. 19.
1.3 Volumetric Meshing
In the final step of our process, we filled in the surface mesh for each cell with tetrahedrons using the Gmsh software tool (Geuzaine and Remacle 2009). This volumetric meshing employed a 3-D Delaunay refinement algorithm that reduces the instance of tetrahedral slivers, making the tetrahedrons more equilateral, thereby improving the mesh quality from the point of view of the finite-element method. Note that the volumetric meshing did not alter the associated surface meshes, insuring that the meshes remained conformal. Fully meshed cells are shown in Fig. 25.
Rights and permissions
About this article
Cite this article
Pages, N., Vera-Sigüenza, E., Rugis, J. et al. A Model of \(\hbox {Ca}^{2+}\) Dynamics in an Accurate Reconstruction of Parotid Acinar Cells. Bull Math Biol 81, 1394–1426 (2019). https://doi.org/10.1007/s11538-018-00563-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-00563-z