Abstract
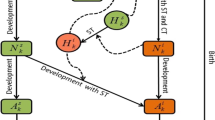
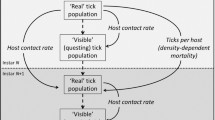
We formulate and analyse a stochastic epidemic model for the transmission dynamics of a tick-borne disease in a single population using a continuous-time Markov chain approach. The stochastic model is based on an existing deterministic metapopulation tick-borne disease model. We compare the disease dynamics of the deterministic and stochastic models in order to determine the effect of randomness in tick-borne disease dynamics. The probability of disease extinction and that of a major outbreak are computed and approximated using the multitype Galton–Watson branching process and numerical simulations, respectively. Analytical and numerical results show some significant differences in model predictions between the stochastic and deterministic models. In particular, we find that a disease outbreak is more likely if the disease is introduced by infected deer as opposed to infected ticks. These insights demonstrate the importance of host movement in the expansion of tick-borne diseases into new geographic areas.





Similar content being viewed by others
References
Allen LJS (2008) An introduction to stochastic epidemic models. In: Brauer F, van den Driessche P, Wu J (eds) Mathematical epidemiology. Springer, Berlin, pp 77–128
Allen LJS (2010) An introduction to stochastic processes with applications to biology, 2nd edn. Chapman and Hall/CRC Press, Boca Ratton
Allen LJS (2012) Branching processes. Encyclopedia of theoretical ecology. University of California Press, Berkeley
Allen LJS (2015) Stochastic population and epidemic models: persistence and extinction. Mathematical biosciences institute lecture series, stochastics in biological systems, vol 1.3. Springer International Publishing, Berlin
Allen LJS, Burgin AM (2000) Comparison of deterministic and stochastic SIS and SIR models in discrete time. Math Biosci 163:1–33
Allen LJS, Lahodny GE Jr (2012) Extinction thresholds in deterministic and stochastic epidemic models. J Biol Dyn 6:590–611
Allen LJS, van den Driessche P (2013) Relations between deterministic and stochastic thresholds for disease extinction in continuous- and discrete-time infectious disease models. Math Biosci 243:99–108
Anderson BE, Sims KG, Olson JG, Childs JE, Piesman JF, Happ CM, Maupin GO, Johnson BJB (1993) Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg 49:239–244
Awerbuch TE, Sandberg S (1995) Trends and oscillations in tick population dynamics. J Theor Biol 175:511–516
Ball F (1983) The threshold behaviour of epidemic models. J Appl Probab 20:227–241
Ball F, Donnelly P (1995) Strong approximations for epidemic models. Stoch Process Appl 55:1–21
Bartlett MS (1956) Deterministic and stochastic models for recurrent epidemics. In: Proceedings of the third Berkeley symposium mathematical statistics and probability, vol 4. University of California Press, Berkeley, pp 81–109
Bartlett MS (1960) Stochastic population models. Methuen, London
Bartlett MS (1964) The relevance of stochastic models for large-scale epidemiological phenomena. Appl Stat 13:2–8. doi:10.2307/2985217
CDC (2015) Tick-borne diseases of the United States: a reference manual for health care providers, 3rd edn. CDC Stacks-Public Health Publications, Atlanta
Dantas-Torres F, Chomel BB, Otranto D (2012) Ticks and tick-borne diseases: a one health perspective review. Trends Parasitol 28:437–446
Das A, Lele SR, Glass GE, Shields T, Petz J (2002) Modelling a discrete spatial response using generalized linear mixed models: application to Lyme disease vectors. Int J Geogr Inf Sci 16:151–166
Diekmann O, Heesterbeek JA, Metz JA (1990) On the definition and computation of the basic reproduction ratio \(R_0\) in models for infectious diseases in heterogeneous populations. J Math Biol 28(4):365–382
Ding W (2007) Optimal control on hybrid ODE systems with application to a tick disease model. Math Biosci Eng 4:633–659
Furman DP, Loomis EC (1984) The ticks of California (Acari: Ixodida). Bulletin of the California Insect Survey, vol 25. University of California Press, Berkeley
Gaff H (2011) Preliminary analysis of an agent-based model for a tick-borne disease. Math Biosci Eng 8:463–473
Gaff HD, Gross JL (2007) Modeling tick-borne disease: a metapopulation model. Bull Math Biol 69:265–288
Gaff HD, Nadolny R (2013) Identifying requirements for the invasion of a tick species and tick-borne pathogen through TICKSIM. Math Biosci Eng 10:625–635
Ghosh M, Pugliese A (2004) Seasonal population dynamics of ticks, and its influence on infection transmission: a semi-discrete approach. Bull Math Biol 66:1659–1684
Glass GE, Schwartz BS, Morgan JM, Johnson DT, Noy PM, Israel E (1995) Environmental risk factors for Lyme disease identified with geographic information systems. Am J Public Health 85:944–948
Hethcote HW (2000) The mathematics of infectious diseases. SIAM Rev 42(4):599–653
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129:S3–S14
Karlin S, Taylor H (1975) A first course in stochastic processes, 2nd edn. Academic Press, New York
Khan A, Hassan M, Imran M (2013) The effects of a backward bifurcation on a continuous time Markov Chain model for the transmission dynamics of single strain dengue virus. Appl Math 4:663–674. doi:10.4236/am.2013.44091
Lahodny GE Jr, Allen LJS (2013) Probability of a disease outbreak in stochastic multipatch epidemic models. Bull Math Biol doi:10.1007/s11538-013-9848-z
Lahodny GE, Gautam R, Ivanek R (2015) Estimating the probability of an extinction or major outbreak for an environmentally transmitted infectious disease. J Biol Dyn 9:128–155
Lloyd AL, Zhang J, Root AM (2007) Stochasticity and heterogeneity in host-vector models. J R Soc Interface 4:851–863
Maliyoni M, Mwamtobe PMM, Hove-Musekwa SD, Tchuenche JM (2012) Modelling the role of diagnosis, treatment, and health education on multidrug-resistant tuberculosis dynamics. ISRN: Biomath doi:10.5402/2012/459829
Masika PJ, Sonandi A, van Averbeke W (1997) Tick control by small-scale cattle farmers in the central Eastern Cape Province, South Africa. J S Afr Vet Assoc 68:45–48
McCormack RK, Allen LJS (2005) Disease emergence in deterministic and stochastic models for host and pathogen. Appl Math Comput 168:1281–1305
Mount GA, Haile DG (1989) Computer simulation of population dynamics of the American dog tick (Acari: Ixodidae). J Med Entomol 26:60–76
Mount GA, Haile DG, Barnard DR, Daniels E (1993) New version of LSTSIM for computer simulation of Amblyomma americanum (Acari: Ixodidae) population dynamics. J Med Entomol 30:843–857
Mount GA, Haile DG, Daniels E (1997) Simulation of management strategies for the blacklegged tick (Acari: Ixodidae) and the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol 90:672–683
Piesman J, Eisen L (2008) Prevention of tick-borne diseases. Annu Rev Entomol 53:323–343
Randolph S (1999) Epidemiological uses of a population model for the tick Rhipicephalus appendiculatus. Trop Med Int Health 4:A34–A42
Sandberg S, Awerbuch TE, Spielman A (1992) A comprehensive multiple matrix model representing the life cycle of the tick that transmits the age of Lyme disease. J Theor Biol 157:203–220
Van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
Whittle P (1955) The outcome of a stochastic epidemic: a note on Bailey’s paper. Biometrika 42:116–122
Wu X, Duvvuri VRSK, Wu J (2010) Modelling the dynamical temperature influence on tick Ixodes scapularis population. International Environmental Modelling and Software Society, International Congress on Environmental Modelling and Software
Acknowledgements
MM thanks the University of Malawi (Chancellor College) for financial support. KSG thanks the National Research Foundation of South Africa and the University of KwaZulu-Natal for ongoing support. The authors are grateful to two anonymous reviewers for their valuable suggestions which improved the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maliyoni, M., Chirove, F., Gaff, H.D. et al. A Stochastic Tick-Borne Disease Model: Exploring the Probability of Pathogen Persistence. Bull Math Biol 79, 1999–2021 (2017). https://doi.org/10.1007/s11538-017-0317-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-017-0317-y