Abstract
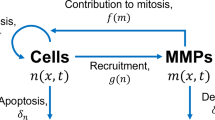
The process of wound healing is governed by complex interactions between proteins and the extracellular matrix, involving a range of signaling pathways. This study aimed to formulate, quantify, and analyze a mathematical model describing interactions among matrix metalloproteinases (MMP-1), their inhibitors (TIMP-1), and extracellular matrix in the healing of a diabetic foot ulcer. De-identified patient data for modeling were taken from Muller et al. (Diabet Med 25(4):419–426, 2008), a research outcome that collected average physiological data for two patient subgroups: “good healers” and “poor healers,” where classification was based on rate of ulcer healing. Model parameters for the two patient subgroups were estimated using least squares. The model and parameter values were analyzed by conducting a steady-state analysis and both global and local sensitivity analyses. The global sensitivity analysis was performed using Latin hypercube sampling and partial rank correlation analysis, while local analysis was conducted through a classical sensitivity analysis followed by an SVD-QR subset selection. We developed a “local-to-global” analysis to compare the results of the sensitivity analyses. Our results show that the sensitivities of certain parameters are highly dependent on the size of the parameter space, suggesting that identifying physiological bounds may be critical in defining the sensitivities.











Similar content being viewed by others
References
Alon U (2006) An introduction to systems biology: design principles of biological Circuits. Chapman & Hall/CRC mathematical and computational biology. Taylor & Francis, London
Blower SM, Dowlatabadi H (1994) Sensitivity and uncertainty analysis of complex models of disease transmission: an hiv model, as an example. Int Stat Rev 62(2):229–243
Bode W, Fernandez-Catalan C, Grams F, Gomis-Rüth FX, Nagase H, Tschesche H, Maskos K (1999) Insights into MMP–TIMP interactions. Ann N Y Acad Sci 878:73–91
Conover WJ, Iman RL (1981) Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat 35(3):124–129. doi:10.1080/00031305.1981.10479327
Eisenberg MC, Hayashi MA (2014) Determining identifiable parameter combinations using subset profiling. Math Biosci 256:116–126. doi:10.1016/j.mbs.2014.08.008
Ellwein LM, Tran HT, Zapata C, Novak V, Olufsen MS (2008) Sensitivity analysis and model assessment: mathematical models for arterial blood flow and blood pressure. J Cardiovasc Eng 8:94–108
Eslami M (1994) Theory of sensitivity in dynamic systems: an introduction. Springer, Berlin
Frank PM (1978) Introduction to system sensitivity theory. Academic Press, New York
Geris L, Schugart RC, van Oosterwyck H (2010) In silico design of treatment strategies in wound healing and bone fracture healing. Philos Trans A Math Phys Eng Sci 368(1920):2683–2706
Golub GH, van Loan CF (1989) Matrix computations, 2nd edn. The Johns Hopkins University Press, Baltimore
Hamby DM (1994) A review of techniques for parameter sensitivity analysis of environmental models. Environ Monit Assess 32(2):135–154
Helton JC, Davis FJ (2002) Illustration of sampling-based methods for uncertainty and sensitivity analysis. Risk Anal 22(3):591–622. doi:10.1111/0272-4332.00041
Iman RL, Conover WJ (1979) The use of the rank transform in regression. Technometrics 21(4):499–509. doi:10.1080/00401706.1979.10489820
International Atomic Energy Agency (1989) Evaluating the reliability of predictions made using environmental transfer models. International Atomic Energy Agency
Jørgensen LN (2003) Collagen deposition in the subcutaneous tissue during wound healing in humans: a model evaluation. APMIS Suppl 115:1–56
Lawrence WT (1998) Physiology of the acute wound. Clin Plast Surg 25(3):321–340
Li M, Moeen Rezakhanlou A, Chavez-Munoz C, Lai A, Ghahary A (2009) Keratinocyte-releasable factors increased the expression of MMP1 and MMP3 in co-cultured fibroblasts under both 2d and 3d culture conditions. Mol Cell Biochem 332(1–2):1–8
Lobmann R, Schultz G, Lehnert H (2005) Proteases and the diabetic foot syndrome: mechanisms and therapeutic implications. Diabetes Care 28(2):461–471
Marino S, Hogue IB, Ray CJ, Kirschner DE (2008) A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol 254(1):178–196. doi:10.1016/j.jtbi.2008.04.011
Maskos K (2005) Crystal structures of MMPs in complex with physiological and pharmacological inhibitors. Biochimie 87(3–4):249–263
Mast B (1992) The skin. In: Cohen IK, Diegelmann RF, Lindblad WJ (eds) Wound healing: biochemical and clinical aspects, Chap. 22. W. B. Saunders Co., London
MathWorks: How globalsearch and multistart work—matlab & simulink.http://www.mathworks.com/help/gads/how-globalsearch-and-multistart-work.html
McKay MD, Beckman RJ, Conover WJ (1979) Comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 21(2):239–245
Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY (2008) Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med 25(4):419–426
Olufsen MS, Ottesen JT (2013) A practical approach to parameter estimation applied to model predicting heart rate regulation. J Math Bio 67(1):39–68
Parks WC, Wilson CL, López-Boado YS (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4(8):617–629
Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC (1997) The activity of collagenase-1 is required for keratinocyte migration on a type 1 collagen matrix. J Cell Biol 137:1445–1457
Pope SR, Ellwein LM, Zapata CL, Novak V, Kelley CT, Olufsen MS (2009) Estimation and identification of parameters in a lumped cerebrovascular model. Math Biosci Eng 6(1):93–115
Sawicki G, Marcoux Y, Sarkhosh K, Tredget EE, Ghahary A (2005) Interaction of keratinocytes and fibroblasts modulates the expression of matrix metalloproteinases-2 and -9 and their inhibitors. Mol Cell Biochem 269(1–2):209–216
Sheehan P, Jones P, Caselli A, Giurini JM, Veves A (2003) Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 26(6):1879–1882
Sherratt JA, Dallon JC (2002) Theoretical models of wound healing: past successes and future challenges. C R Biol 325(5):557–564
Stamenkovic I (2003) Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 200:448–464
Stein M (1987) Large sample properties of simulations using latin hypercube sampling. Technometrics 29(2):143–151. doi:10.1080/00401706.1987.10488205
Valdez-Jasso D, Haider MA, Banks HT, Bia D, Zocalo Y, Armentano R, Olufsen MS (2008) Viscoelastic mapping of the arterial ovine system using a kelvin model. IEEE Trans Biomed Eng 56:210–219
Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB (1987) Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA 84(16):5788–5792
Werner S, Krieg T, Smola H (2007) Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol 127(5):998–1008
Yager DR, Kulina RA, Gilman LA (2007) Wound fluids: a window into the wound environment? Int J Low Extrem Wounds 6(4):262–272
Zhang Q, Gould LJ (2014) Hyperbaric oxygen reduces matrix metalloproteinases in ischemic wounds through a redox-dependent mechanism. J Invest Dermatol 134:237–246
Acknowledgments
NK was supported under a Gatton Academy Research Internship Grant. HP was supported by a Western Kentucky University (WKU) Faculty-Undergraduate Student Engagement (FUSE) Award #14-SP141, a WKU Honors Development grant, and a Gatton Alumni Scholarship Award. CC was supported by a National Science Foundation Research Experiences for Undergraduates (REU) grant DBI-1004665. RS was partially supported as a Sabbatical Fellow at the National Institute for Mathematical and Biological Synthesis, an Institute sponsored by the National Science Foundation through NSF Award #DBI-1300426, with additional support from The University of Tennessee, Knoxville. Additional financial support was also provided by the WKU Office of Sponsored Programs, the WKU Office of Academic Affairs, the WKU Office of Research, the WKU Ogden College Dean’s Office, the WKU Biotechnology Center, the WKU Bioinformatics Science Center, the WKU Applied Research and Technology Program, the WKU Biology and Mathematics Departments, the University of Chicago Department of Mathematics, and the Gatton Academy of Mathematics and Science in Kentucky. Permission was granted by Wiley to use the data given in Muller et al. as “reuse of this article is permitted in accordance with the Creative Commons Deed, Attribution 2.5, which does not permit commercial exploitation.”
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1.1 Steady-State Analysis
Equations (11–14) were first rewritten to be functions of the states present in the equations,
The steady states, denoted by \({\overline{\mathbf{x}}} = (\overline{M},\overline{T},\overline{E},\overline{f})\), are the solutions to Eqs. (22–25) when the derivatives are set to 0. Only nonnegative solutions were considered. The stability of each steady state was determined by computing the eigenvalues of the Jacobian matrix (26) evaluated at the steady state.
Evaluating (26) results in a matrix whose only nonzero element in the third column is \(\partial r/\partial E\) and only nonzero element in the fourth row is \(\partial s/\partial f\). Therefore, the eigenvalues of (26) are the two partial derivatives and the eigenvalues of the \(2 \times 2\) block matrix in the upper left-hand corner,
We note that for any \(\overline{M}\) and \(\overline{f}\),
The derivative
will be looked at in the proceeding cases. Nonzero solutions for \(\overline{M}\) and \(\overline{T}\) cannot be found explicitly in terms of model parameters and other states. The general cases are analyzed for stability and, if possible, existence.
Case 1: \(\overline{f} = 0\). Mathematically and biologically, this is the simplest case. The only steady-state vector \({\overline{\mathbf{x}}}\) with all nonnegative components for this case is \((\overline{M}, \overline{T}, \overline{E}, \overline{f}) = (0, 0, 0, 0)\). The eigenvalues of the Jacobian matrix (26) evaluated at this state are \(-k_3\), \(-k_7\), \(-k_{10}\), and \(k_{11} - k_{12}\), so \({\overline{\mathbf{x}}}\) is stable when \(k_{11} < k_{12}\) (when the growth rate of fibroblasts is less than the death rate). This steady state corresponds to a completely unhealed, nonviable tissue condition, with ECM levels at 0. We conclude that the growth rate of fibroblasts must be strictly greater than the decay rate for any healing activity to occur.
Case 2: \(\overline{M} = 0, \overline{f} \ne 0\). Solving for the other states gives \(\overline{E} = (\overline{f}k_8)/(k_{10}+\overline{f}k_8)\), \(\overline{T} = 0\), and the nonzero \(\overline{f}\) given in (29). The eigenvalues of (26) evaluated at this state are \(-k_3,-k_7\), and the derivatives given in Eqs. (30, 31). Plugging \(\overline{f}\) into (31), we see that when \(k_{11} > k_{12}\), \({\overline{\mathbf{x}}} = (0,0,\overline{E},\overline{f})\) is a stable steady state. Noting that if \(k_{11} < k_{12}\) the nonzero \(\overline{f}\) is negative, we conclude that this steady state is stable if it exists. Biologically, this state corresponds to reduced MMP and TIMP activity over time. Wound closure is also closest to 100 % in this case, as \(\overline{M} = 0\), making \(\overline{E}\) at its maximum in Eq. (28). MMPs are present at low levels except at times of wound healing, so initial activity followed by low concentrations would indicate a healed wound. We conclude that this is the healthiest end state.
Case 3: \(\overline{M} \ne 0, \overline{T} = 0,\overline{f} \ne 0\). Rewriting (22) as a polynomial by plugging in \(\overline{T}=0\) and multiplying by \((-k_2^3-M^3)/M\),
With this notation, the positive zeros of \(\varphi \) are the \(\overline{M}\) values of the steady states. It can be checked that \(\varphi '(M) = 0\) when \(M = 0\) or \(2\overline{f}k_1/(3k_3)\). The second root of \(\varphi '(M)\) corresponds to the relative minimum of \(\varphi (M)\). Since \(\varphi (0) > 0\), positive roots exist if and only if the relative minimum is less than or equal to zero.
It follows that a steady state exists only if
To assess the stability of the steady state(s), assume the condition holds. If \(\varphi (\overline{M}) = 0\), then by Eq. (32),
The eigenvalues of (26) evaluated at \(\overline{\mathbf{x}} = (\overline{M},0,\overline{E},\overline{f})\) are the diagonal entries. The entries in the second, third, and fourth rows are negative. Substituting the equality in Eq. (35) and evaluating the eigenvalue \(\lambda \) give
The expression is negative if and only if \(\varphi '(\overline{M}) > 0\). It follows that if the condition in Eq. (34) is an equality, then the steady state is unstable. In the case where the condition is a strict inequality, the larger of the two roots is stable.
Case 4: \(\overline{M} \ne 0\), \(\overline{T} \ne 0\), \(\overline{f} \ne 0\) For this case, \(\overline{M}\) and \(\overline{T}\) cannot be easily found algebraically. Necessary conditions for existence of the nullclines of (22) and (23) in the first quadrant are established to ensure that intersections can occur when \(M > 0\) and \(T>0\). Solving for T in \(p / M=0\) and M in \(q / T=0\) gives the nullclines of (22) and (23), respectively,
The y-intercept of g(M) is \(\frac{-k_3}{k_4}<0\) and \(\displaystyle \lim _{M \rightarrow \infty } g(M)=\frac{-k_3}{k_4}<0\). Because g(M) is a continuous function over the interval \([0, \infty )\) the relative maximum in this interval must be greater than 0 for existence in the first quadrant. The only relative maximum value in the domain \([0, \infty )\) is \(\frac{2^{2 / 3} \overline{f} k_1-3k_2 k_3}{3k_2 k_4} \). Thus, the condition for the existence of the nullcline (37) in the first quadrant is
The y-intercept of h(T) is \(\frac{-k_7}{k_4}<0\) and \(\lim \limits _{T \rightarrow \infty } h(T)=\frac{-k_7}{k_4}<0\). The function is continuous and negative over the domain \(T \in [0, \infty )\) unless there is a discontinuity of the second kind resulting from a zero in the denominator. By imposing a condition such that there are exactly two zeros in the denominator in \([0, \infty )\), at least one part of the graph will be in the first quadrant. Let
Routine computation shows that \(\phi '(T) = 0\) when \(T = 0\) or \(2fk_5 / (3 k_4)\). Noting that \(\phi (0) < 0\) and that \(\phi \) has positive roots if and only if the relative maximum of \(\phi (T)\) is greater than 0,
It follows that h(T) has discontinuities if and only if
Conditions (39) and (42) are necessary conditions for the nullclines’ existence in the first quadrant and are not sufficient conditions for the existence of the steady state(s). To check for stability, we note that the real parts of the eigenvalues of a 2x2 matrix are negative if and only if the trace is negative and determinant is positive. The trace and determinant of matrix (27) are
Setting the trace to be less than 0 and the determinant to be greater than 0, two conditions for stability are found:
Both Cases 3 and 4 represent a less complete healing response since long-term MMP activity corresponds to a lower \(\overline{E}\) compared to Case 2.
Rights and permissions
About this article
Cite this article
Krishna, N.A., Pennington, H.M., Coppola, C.D. et al. Connecting Local and Global Sensitivities in a Mathematical Model for Wound Healing. Bull Math Biol 77, 2294–2324 (2015). https://doi.org/10.1007/s11538-015-0123-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-015-0123-3